Chemistry is an amazing science. So many incredible things can be found in seemingly ordinary things. Everything material that surrounds us everywhere exists in several states of aggregation: gases, liquids and solids. Scientists have also identified the 4th - plasma. At a certain temperature, a substance can change from one state to another. For example, water: when heated above 100, from liquid form it turns into steam. At temperatures below 0, it transforms into the next aggregate structure - ice.
…
The entire material world contains a mass of identical particles that are interconnected. These smallest elements are strictly lined up in space and form the so-called spatial frame.
This is interesting: anions and cations in chemistry, solubility table.
What is graphite atomic crystal lattice?
The table below lists the characteristic properties of substances with atomic and ionic crystal lattices.
Characteristic properties of substances
- solid under normal conditions;
- conduct electric current in melts and solutions
| With atomic crystal lattice | With ionic crystal lattice |
Using this information, determine what kind of crystal lattice it has:
1) calcium chloride
;
2) graphite
.
Write your answer in the space provided:
1) Calcium chloride has
Calcium chloride is a substance with an ionic chemical bond, refractory (T mp = 772 °C), conducts electric current - has an ionic crystal lattice.
Graphite is a substance with a covalent nonpolar chemical bond, nonvolatile, solid, and has an atomic crystal lattice.
Answer: Calcium chloride is an ionic crystal lattice, graphite is an atomic crystal lattice.
Source
Table of types of crystal lattices: iodine, diamond, graphite, sodium
Chemistry is an amazing science.
So many incredible things can be found in seemingly ordinary things. Everything material that surrounds us everywhere exists in several states of aggregation: gases, liquids and solids. Scientists have also identified the 4th - plasma. At a certain temperature, a substance can change from one state to another. For example, water: when heated above 100, from liquid form it turns into steam. At temperatures below 0, it transforms into the next aggregate structure - ice.
The entire material world contains a mass of identical particles that are interconnected. These smallest elements are strictly lined up in space and form the so-called spatial frame.
This is interesting: anions and cations in chemistry, solubility table.
Definition
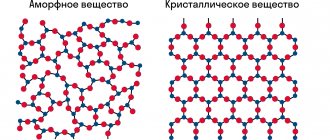
A crystal lattice is a special structure of a solid substance in which the particles stand in a geometrically strict order in space.
In it you can find nodes - places where elements are located: atoms, ions and molecules and internodal space. Solids , depending on the range of high and low temperatures, are crystalline or amorphous - they are characterized by the absence of a certain melting point. When exposed to elevated temperatures, they soften and gradually turn into liquid form. These types of substances include: resin, plasticine.
In this regard, it can be divided into several types:
But at different temperatures, one substance can have different forms and exhibit diverse properties. This phenomenon is called allotropic modification.
This is interesting: metals and non-metals in the periodic table of Mendeleev.
Therapeutic effect
Graphite has a large number of healing properties, which allows folk medicine to actively use it as a remedy for diseases.
- Graphite has a beneficial effect on the layers of the epidermis. It is used in the treatment of skin cracks, scars, bruises, and eczema.
- Lithotherapists use graphite products to treat diseases in the sinus area. The stone helps get rid of dryness in the nasal mucosa, eliminate various chronic diseases of the respiratory tract, rhinitis, laryngotracheitis. Graphite is also used as a prophylactic treatment for bronchial asthma.
- Graphite stones help improve the condition of the gastrointestinal tract, improve metabolic processes in the body, fight chronic gastritis, eliminate heartburn and reduce the worsening of constipation or diarrhea.
- Healing graphite helps cope with severe headaches, apathy and stress.
- Graphite has a healing effect on human eyes. Small stones relieve inflammation in barley, conjunctivitis, and also help in the treatment of cataracts or corneal ulcers.
- The mineral regulates emotional balance, reducing the level of anger, temper, aggression, depression and neurasthenia.
Metal type
Its structure resembles a molecular one, but it still has stronger bonds. The difference between this type is that its nodes contain positively charged cations. Electrons that are located in the interstitial space participate in the formation of the electric field.
They are also called electric gas. Simple metals and alloys are characterized by a metal lattice type. They are characterized by the presence of a metallic luster, plasticity, thermal and electrical conductivity. They can melt at different temperatures.
Source
MORPHOLOGY
Well-formed crystals are rare. The crystals are lamellar, scaly, curved, usually of an imperfect lamellar shape. Most often these are leaves without crystallographic outlines and their aggregates. Forms continuous radial-radial aggregates, cryptocrystalline, sheet or round, less often - spherulite aggregates of concentric-zonal structure. In coarse-crystalline sediments, triangular shading is often observed on the (0001) planes.
Crystal lattices
The crystal lattice is the spatial arrangement of atoms or ions in a crystal. The points of the crystal lattice at which atoms or ions are located are called lattice nodes.
Crystal lattices are divided into molecular, atomic, ionic and metallic.
It is very important not to confuse the type of chemical bond and crystal lattice. Remember that crystal lattices reflect the spatial arrangement of atoms.
Molecular crystal lattice
Molecules are located at the nodes of a molecular lattice. Under normal conditions, most gases and liquids have a molecular lattice. Bonds are most often covalent polar or nonpolar.
The classic example of a substance with a molecular lattice is water, so associate the properties of these substances with water. Substances with a molecular lattice are fragile, have low hardness, are volatile, fusible, capable of sublimation, and are characterized by low boiling points.
Examples: NH3, H2O, Cl2, CO2, N2, Br2, H2, I2. I would especially like to mention white phosphorus, orthorhombic, plastic and monoclinic sulfur, and fullerene. We studied these allotropic modifications in detail in an article devoted to the classification of substances.
Ionic crystal lattice
At the sites of the ionic lattice there are atoms connected by ionic bonds. This type of lattice is characteristic of substances that have ionic bonds: metal salts, oxides and hydroxides.
Metal crystal lattice
The nodes of the metal lattice contain metal atoms. This type of lattice is characteristic of substances formed by a metallic bond.
Associate the properties of these substances with copper. They have a characteristic metallic luster, are malleable and ductile, conduct electricity and heat well, and have high melting and boiling points.
Examples: Cu, Fe, Zn, Al, Cr, Mn.
Atomic crystal lattice
At the sites of the atomic lattice there are atoms connected by covalent polar or nonpolar bonds.
Associate these substances with sand. They are very hard, very refractory (high melting point), non-volatile, durable, and insoluble in water.
Examples: SiO2, B, Ge, SiC, Al2O3. I would especially like to highlight: diamond and graphite (C), red and black phosphorus (P).
© Bellevich Yuri Sergeevich 2018-2021
This article was written by Yuri Sergeevich Bellevich and is his intellectual property. Copying, distribution (including by copying to other sites and resources on the Internet) or any other use of information and objects without the prior consent of the copyright holder is punishable by law. To obtain article materials and permission to use them, please contact Yuri Bellevich
.
Source
Description of graphite:
Graphite is widely distributed in nature as a mineral. It is usually found in the form of individual scales, plates and clusters, varying in size and content.
Natural graphite is not pure in its chemical composition. It contains large quantities (up to 10-25%) of ash, consisting of various components (Fe2O3, SiO2, Al2O3, MgO, P2O5, CuO, CaO, etc.), gases (up to 2%) and bitumen, and sometimes water.
Graphite is also obtained artificially in various ways. For example, by heating a mixture of coke and pitch to 2,800 °C.
ORIGIN
It forms at high temperatures in volcanic and igneous rocks, pegmatites and skarns. It occurs in quartz veins with wolframite and other minerals in mid-temperature hydrothermal polymetallic deposits. Common in metamorphic rocks - crystalline schists, gneisses, marbles. Large deposits are formed as a result of coal pyrolysis under the influence of traps in coal deposits (Tunguska basin). Accessory mineral of meteorites. Associated minerals: quartz, pyrite, garnets, spinel.
Types and grades of graphite:
In accordance with GOST 17022-81 “Graphite. Types, grades and general technical requirements" distinguishes the following mineralogical types of graphite:
The same GOST provides for the following grades of graphite: GSM-1, GSM-2, GAK-1, GAK-2, GAK-3, GK-1, GK-2, GK-3, GS-1, GS-2, GS- 3, GS-4, P, EUZ-M, EUZ-II, EUZ-III, EUT-I, EUT-II, EUT-III, GT-1, GT-2, GT-3, GE-1, GE- 2, GE-3, GE-4, GL-1, GL-2, GL-3, EUN, GLS-1, GLS-2, GLS-3, GLS-4.
The following types of use (consumption) of graphite correspond to them:
– special low-ash graphite,
– crystalline electrocarbon graphite,
– crystalline casting graphite,
– cryptocrystalline electrocarbon graphite,
– cryptocrystalline casting graphite.
Structure and crystal lattice of graphite:
Graphite has a layered, flat structure. The individual layers of graphite are called graphene. Each layer of the graphite crystal lattice can be positioned differently in relation to each other, forming polytypes.
In each layer, carbon atoms are arranged in a hexagonal lattice at a distance of 0.142 Nm, and the distance between graphene planes is 0.335 Nm.
Carbon atoms located in the same plane of the layer are connected to each other by a covalent bond. Carbon has four free electrons. However, a covalent bond involves only three of the four electrons, so each carbon atom is bonded to only three carbon atoms. The fourth electron migrates freely in the plane, making the graphite electrically conductive in a direction parallel to the plane. The electrical conductivity of graphite in the direction perpendicular to the plane of the layer, on the contrary, is hundreds of times less.
The graphene layers in graphite are held together by weak van der Waals forces, which allow the graphite layers to be easily separated from each other.
There are two known forms of graphite: alpha graphite (has a hexagonal structure and crystal lattice) and beta graphite (has a rhombohedral structure and crystal lattice). Both forms of graphite have very similar physical properties, except that the graphene layers of each form of graphite are stacked slightly differently.
Rice. 1. Alpha graphite
In α-graphite, half of the atoms of each layer are located above and below the centers of the hexagon, and in β-graphite, every fourth layer repeats the first.
Rice. 2. Beta graphite
Alpha graphite can be converted to beta form by mechanical processing. The beta form transforms into the alpha form when graphite is heated above 1300 °C.
What can graphite and diamond be like?
The appearance of graphite in any of its forms remains almost the same. It is gray, can be almost black, and has a metallic sheen. However, it cannot be classified as a metal; carbon cannot be one. Under pressure, graphite always disintegrates into flakes, and its hardness can vary. However, in any case, it cannot be compared with a diamond.
When examining a diamond, that is, a cut diamond, one can notice its transparency - although some stones are still cloudy, and they can also have different shades. But diamond powder will always be white , while graphite powder remains black or gray. Despite all the abundance of natural variations, these two forms of carbon are always very different and cannot be confused.
Properties of graphite:
– the electrical conductivity of graphite is anisotropic (that is, it depends on the direction inside the graphite itself). It conducts electric current well in a direction parallel to the base plane. In this case, its electrical conductivity is close to metallic. In the perpendicular direction, electrical conductivity is hundreds of times less.
– has low hardness. Mohs hardness 1.
– relatively soft. After exposure to high temperatures it becomes slightly harder and very brittle,
– density 2.08-2.23 g/cm³,
– easy to machine,
– color from iron-black to steel-gray, metallic luster,
– greasy (slippery) to the touch, leaves a mark on paper and fingers,
– during friction, graphite exfoliates into separate flakes (this property is used in pencils),
– has a fairly high thermal conductivity. The thermal conductivity of graphite is anisotropic. It ranges from 100 to 354.1 W/(m*K) and depends on the grade of graphite, on the direction relative to the basal planes and on temperature,
– the coefficient of thermal expansion of graphite is also anisotropic and depends on temperature. Up to 700 K, the coefficient of thermal expansion of graphite is negative in the direction of the basal planes (graphite contracts when heated), its absolute value decreases with increasing temperature. Above 700 K the coefficient of thermal expansion becomes positive. In the direction perpendicular to the basal planes, the coefficient of thermal expansion is positive, practically independent of temperature and more than 20 times higher than the average absolute value for the basal planes,
– has high diamagnetism,
– chemically inactive,
– has chemical resistance. Acid resistant
– at high temperatures reacts with oxygen, burning to carbon dioxide,
– forms inclusion compounds with alkali metals and salts.
Source
Obtaining graphite
: graphite is obtained by heating anthracite without access to air.
APPLICATION
For the manufacture of melting crucibles, lining plates - the application is based on the high heat resistance of graphite (in the absence of oxygen), its chemical resistance to a number of molten metals. It is used in electrodes and heating elements due to its high electrical conductivity and chemical resistance to almost all aggressive aqueous solutions (much higher than that of precious metals). For the production of chemically active metals by electrolysis of melts, solid lubricants, combined liquid and paste lubricants, plastic fillers.
it is a neutron moderator in nuclear reactors, a component of a composition for the production of rods with black graphite (mixed with kaolin). It is used to obtain synthetic diamonds as a nanometer length standard for the calibration of tunnel effect microscope and atomic force microscope scanners, for the manufacture of contact brushes and current collectors for various electrical machines, electric vehicles and trolley overhead cranes, high-power rheostats and other devices where required reliable mobile electrical contact for the manufacture of thermal protection for the nose of ballistic missile warheads and re-entry spacecraft.
Graphite - Before
| Molecular mass | 12.01 g/mol |
| origin of name | from ancient Greek. - write write |
| IMA status | valid, first described before 1959 (pre-IMA) |