Not everyone knows, but diamond and graphite are two forms of the same substance. These minerals are completely different from each other in hardness and in the characteristics of refraction and reflection of light. Moreover, the differences are quite significant. Diamond is the hardest mineral in the world, on the Mohs scale it represents a standard of 10, while the hardness of graphite on this scale is only 2. Thus, diamond and graphite are simultaneously the most similar and dissimilar substances in the world.
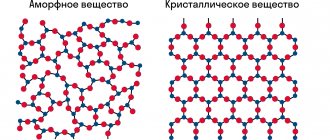
Crystal lattices of diamond and graphite
Each of them comes from carbon, which, in turn, is the most abundant element in the biosphere. It is present both in the atmosphere and in water, in biological objects. In the ground it is present in the composition of oil, gas, peat, and so on. It is also found as deposits of graphite and diamond.
Most carbon is found in organisms. Moreover, none of them can do without it. And the origin of this mineral in other parts of the planet is precisely explained by the presence of living organisms once there.
Much controversy surrounds the question of where graphite and diamonds came from, because it is not enough for there to be carbon alone; it is also necessary that certain conditions be met under which this chemical element takes on a new structure. It is believed that the origin of graphite is metamorphic, and diamonds are igneous. This means that the formation of diamonds on the planet is accompanied by complex physical processes, most likely in the deep layers of the earth during combustion and explosions in the presence of oxygen. Scientists suggest that methane is also involved in this process, but no one knows for sure.
Myths and misconceptions about diamond and graphite
For a long time it was believed that diamond and graphite, which are dissimilar to each other, are different substances. In 1797, the chemist S. Tennant conducted a series of experiments in which he compared the composition, proving that they were based on carbon.
When comparing the structure of diamond and graphite at the atomic level, a significant difference is revealed:
- Diamond has a crystal lattice in the shape of a tetrahedron, where each atom is surrounded by 4 other atoms, being the vertex of the neighboring tetrahedron. They are similar to honeycombs, only in 3D. This structure gives strong bonds between the molecules of the substance.
- Graphite is also represented by hexagons, but they are located in horizontal layers, between which there is no strong connection. This makes it soft and pliable.
The properties of diamond and graphite depend on the structure of the crystal lattice, therefore they are radically different; what they have in common is the ability to conduct heat, heating up in the process. If we compare the other indicators, they differ:
- graphite leaves a greasy mark on paper, diamond does not;
- the gem is presented in different colors, its dissimilar “brother” – in one;
- the crystal is the standard of hardness, while the second carbon material crumbles at the slightest pressure;
- areas of use are different.
Thermal conductivity of diamond
The thermal conductivity of diamond [0 35 cal / (cm-sec - C)] is higher than the thermal conductivity of hard alloys [0 05 - 0 19 cal / (cm-sec - C) 1 and high-speed steel [0 05 - 0 07 cal / (cm-sec — C) 1, therefore, the heat generated during cutting is removed from the cutter in a diamond tool faster than in a carbide tool.
Diamond's thermal conductivity is also the highest among minerals, allowing diamond weapons to cool quickly.
This property is important for preventing the destruction of diamonds during rapid heating and thermal cracking.
A decrease in the thermal conductivity of Pa type diamonds caused by irradiation with fast electrons (1.5 MsBV and the associated thermal rnnnn.
The temperature variation of the thermal conductivity of diamonds of various types reveals its peak, which falls in the temperature range 60 - 100 K [340], which, due to the high Debye temperature, is maximally shifted to the region of higher temperatures compared to other crystalline materials.
Unlike single crystals, the thermal conductivity of polycrystalline diamonds has been poorly studied. There are only a few publications on this issue. There are no data on thermal conductivity for natural polycrystalline diamonds.
C diamond is 50% higher than the thermal conductivity of natural diamond, in which the C isotope content is 98 9%, and at room temperature it turned out to be the highest of all materials ever created by nature or man.
The temperature dependence of the thermal conductivity of diamonds is shown in Fig. 12.1.7, and in Fig. Figure 12.1.8 shows the change in thermal conductivity with the concentration of the 13C isotope at a fixed temperature.
Our results, in particular, explain the independence of the thermal conductivity of diamond from temperature - a fact that contradicts the Peierls-Debye theory.
W; ko is the coefficient of heat transfer intensity, depending on the thermal conductivity of diamonds, the matrix and the body of the crown, the geometric dimensions of the crown, the type and consumption of the cleaning agent, the grain size and concentration of bulk diamonds, W / C, t is the temperature of the cleaning agent flow at the approach to the crown, C.
C diamond is 50% higher than the thermal conductivity of natural diamond, in which the C isotope content is 98 9%, and at room temperature it turned out to be the highest of all materials ever created by nature or man. The temperature dependence of the thermal conductivity of diamonds is shown in Fig. 12.1.7, and in Fig. Figure 12.1.8 shows the change in thermal conductivity with the concentration of the 13C isotope at a fixed temperature.
In IR absorption they correspond to spectra containing, respectively, A and B absorption bands in the single-phonon region. This assumption made it possible to satisfactorily explain the results of studying the thermal conductivity of natural nitrogen-containing diamonds.
In IR absorption they correspond to spectra containing, respectively, A and B absorption bands in the single-phonon region. This assumption made it possible to satisfactorily explain the results of studying the thermal conductivity of natural nitrogen-containing diamonds.
Physical and chemical properties of minerals
The chemical formula of diamond and graphite is the same - it is carbon, designated in the periodic table as C. The gem contains impurities of magnesium, iron, nitrogen, aluminum, which gives it color.
The crystal lattice type of diamond is cubic, and that of graphite is hexagonal. This explains that the hardness of diamond is 5 times greater than that of its “brother”.
The structure of diamond is twofold - the mineral is hard, but at the same time brittle. In graphite, which consists of flakes, it is layered. Comparison of the structure gives the answer to the difference in optical properties - the first conducts light through itself, and the second does not.
A comparison of the physical properties of diamond and graphite is presented in the table:
| Criterion | Diamond | Graphite |
| Crystal cell | Cubic | Flat |
| Transparency | Transparent, semi-transparent, less often cloudy | Opaque |
| Electrical conductivity | Absent | good |
| Thermal conductivity | Present | Present |
| Melting temperature | 4000 °C | 3890 °C |
| Color | Colorless, blue, white, yellow, etc. | Grey |
| Density | 3.56 kg/m³ | 2.23 kg/m³ |
| State of aggregation | Solid | Solid |
| Mohs hardness | 10 | 2 |
Ideal and real crystals. Growing Crystals
An ideal crystal is, in fact, a mathematical object that has complete, inherent symmetry, idealized equal faces.
A real crystal always contains various defects, distortions and irregularities on the faces, and has reduced polyhedron symmetry due to growth conditions, heterogeneity of the feeding medium, damage and deformation. A real crystal does not necessarily have faces of the correct shape, but it retains the main property - the regularity of the position of atoms in the crystal lattice. Each crystalline substance has a certain spatial form characteristic of it. For example, for sodium chloride it is a cube, for aluminum alum it is an octahedron. And even if at first such a crystal had an irregular shape, it will still sooner or later turn into a cube or octahedron. Moreover, if a crystal with the correct shape is deliberately damaged - its vertices are knocked off, edges and faces are damaged, then with further growth it will begin to “heal” its damage on its own. To verify this, the following experiment was carried out: a ball was carved out of a crystal of table salt, and then placed in a saturated solution; After some time, the crystal itself gradually took the shape of a cube! But it is practically impossible to achieve the ideal shape of crystals in terrestrial conditions because, when growing, the force of gravity acts on it, which contributes to the deformation of the edges. Therefore, experiments were carried out on growing crystals in zero-gravity conditions on board spacecraft.
How are crystals grown? Here is a simple model that explains the essence of crystallization. Let's imagine that parquet is being laid in a large hall. It is easiest to work with square-shaped tiles - no matter how you turn them, they will still fit in their place. It is more difficult to lay parquet from rectangular planks, especially if they have grooves and protrusions on the sides - then each plank can be laid in its place in only one way. It is especially difficult to lay out a pattern from planks of complex shapes - it resembles assembling a puzzle. If the parquet floorer is in a hurry, the tiles will arrive at the installation site very quickly - the correct pattern will not work, and voids will appear. Approximately the same processes occur during the growth of crystals, only the difficulty lies in the fact that the particles must fit not in a plane, but in space. They continuously perform thermal movements and “look” for the most convenient place for themselves - an energetically favorable position. Once on such a place on the surface of a growing crystal, a particle of the substance can remain there and after some time end up inside, under the new grown layers. But another outcome is also possible - the particle will again leave the surface into the solution and again begin to “search” for where it is more convenient for it to settle.
To grow crystals, conditions are needed: high pressure, vacuum, temperature. Crystals of some substances can be obtained in the school laboratory. To grow copper sulfate crystals, we obtained a saturated solution. They poured it into a glass where the crystal would grow. The water should not cool down, so the glass with the solution was placed in a container with hot water. A crystal was tied to a thread, and it was tied to a rod, which was placed on a glass and the crystal was dipped into the solution. We placed the glass where there are no drafts, vibrations, or strong light. Cover the glass with paper to prevent dust and debris. After 2-3 days the crystal began to grow. When growing you need to remember:
The crystal should not be removed from the solution without a special reason.
Periodically change or refresh the saturated solution.
Copper sulfate forms a diamond-shaped blue crystal, similar to a gemstone.
Application of crystals.
What are crystals for?
The industrial uses of crystals are so numerous and varied that they are difficult to list. Therefore, we will limit ourselves to a few examples. (presentation “application of crystals”)
The hardest and rarest of natural minerals is diamond. Today, a diamond is primarily a working stone, not a decoration stone.
Due to its exceptional hardness, diamond plays a huge role in technology. Diamond saws are used to cut stones. A diamond saw is a large (up to 2 meters in diameter) rotating steel disk, on the edges of which cuts or notches are made. Fine diamond powder mixed with some adhesive substance is rubbed into these cuts. Such a disk, rotating at high speed, quickly saws any stone.
Diamond is of enormous importance when drilling rocks and in mining operations.
Diamond points are inserted into engraving tools, dividing machines, hardness testing apparatus, and drills for stone and metal.
Diamond powder is used to grind and polish hard stones, hardened steel, hard and super-hard alloys. The diamond itself can only be cut, polished and engraved with diamond. The most critical engine parts in automotive and aircraft production are processed with diamond cutters and drills.
Ruby and sapphire are among the most beautiful and most expensive of precious stones. All these stones have other qualities, more modest, but useful. Blood-red ruby and blue-blue sapphire are siblings, they are generally the same mineral - corundum, aluminum oxide A12O3. The difference in color arose due to very small impurities in aluminum oxide: a tiny addition of chromium turns colorless corundum into a blood-red ruby, titanium oxide into sapphire. There are corundums of other colors. They also have a very modest, nondescript brother: brown, opaque, fine corundum - emery used to clean metal, from which sandpaper is made. Corundum, with all its varieties, is one of the hardest stones on Earth, the hardest after diamond. Corundum can be used to drill, grind, polish, sharpen stone and metal. Grinding wheels, whetstones, and grinding powders are made from corundum and emery.
The entire watch industry runs on artificial rubies. In semiconductor factories, the finest circuits are drawn with ruby needles. In the textile and chemical industries, ruby thread guides draw threads from artificial fibers, nylon, and nylon.
The new life of ruby is a laser or, as it is called in science, an optical quantum generator (OQG), a wonderful device of our days. In 1960 The first ruby laser was created. It turned out that the ruby crystal amplifies the light. The laser shines brighter than a thousand suns. A powerful laser beam with enormous power. It easily burns through sheet metal, welds metal wires, burns through metal pipes, and drills the thinnest holes in hard alloys and diamond. These functions are performed by a solid laser using ruby, garnet and neodite. In eye surgery, neodyne lasers and ruby lasers are most often used. Ground-based short-range systems often use gallium arsenide injection lasers.
New laser crystals have also appeared: fluorite, garnets, gallium arsenide, etc.
Sapphire is transparent, so plates for optical instruments are made from it.
The bulk of sapphire crystals goes to the semiconductor industry.
Flint, amethyst, jasper, opal, chalcedony are all varieties of quartz. Small grains of quartz form sand. And the most beautiful, most wonderful variety of quartz is rock crystal, that is, transparent quartz crystals. Therefore, lenses, prisms and other parts of optical instruments are made from transparent quartz.
Crystals played an important role in many technical innovations of the 20th century. Some crystals generate an electrical charge when deformed. Their first significant application was the manufacture of radio frequency oscillators stabilized by quartz crystals. By forcing a quartz plate to vibrate in the electric field of a radio frequency oscillatory circuit, it is possible to stabilize the receiving or transmitting frequency.
Semiconductor devices, which revolutionized electronics, are made from crystalline substances, mainly silicon and germanium. In this case, alloying impurities that are introduced into the crystal lattice play an important role. Semiconductor diodes are used in computers and communications systems, transistors have replaced vacuum tubes in radio engineering, and solar panels placed on the outer surface of spacecraft convert solar energy into electrical energy. Semiconductors are also widely used in AC-DC converters.
Crystals are also used in some masers to amplify microwave waves and in lasers to amplify light waves. Crystals with piezoelectric properties are used in radio receivers and transmitters, in pickup heads and in sonar. Some crystals modulate light beams, while others generate light under the influence of an applied voltage. The list of uses for crystals is already quite long and is constantly growing.
Similarities and mutual transformations of diamond and graphite
Soft gray graphite and hard transparent diamond have a common base - carbon, i.e. they consist of the same substance. This gave scientists the idea of trying to transform graphite into diamond.
You can get a diamond from graphite in the laboratory or at home. In the first case, the following conditions are needed:
- gas environment (methane);
- pressure more than 50 thousand atmospheres;
- temperature above 1200 °C;
- presence of catalysts (platinum, nickel, iron).
For home experimenters, there are 2 ways. The first one will need:
- current source;
- graphite;
- cold water or liquid nitrogen;
- the wire.
The wire is tied to graphite and lowered into the container. After cooling in a freezer or using liquid nitrogen, current is passed through the resulting wire structure. This contributes to the restructuring of the crystal lattice, rapid transformation into a gem.
The second method involves the appearance of a diamond from a mixture of salt, graphite and distilled water. The thread is lowered into a jar with the prepared solution, and a crystal gradually grows on it.
Graphite can be obtained from a crystal by heating it from 1000 °C; at temperatures from 1750 °C the process occurs quickly.
Also see the reverse method of obtaining graphite from artificial diamonds:
Definition
Diamond is a mineral based on carbon. It is characterized by metastability, that is, the ability to exist in an unchanged form under normal conditions for an indefinitely long time. Placing diamond in specific conditions, for example in a vacuum at an elevated temperature, leads to its transition to graphite.
Diamond
Graphite is a mineral that acts as a modification of carbon. During friction, scales are separated from the total mass of the substance. The most famous use of graphite is making pencil leads from it.
Graphite
Applications of carbon minerals
Carbon minerals have a wide range of properties that allow them to be used in different areas of life. You can find them in a ring, a pencil, a device for cutting metal or glass. If you compare the spheres of use, you can see that they intersect only at one point - mutual transformation.
Diamond
The main area of application of minerals is jewelry making. During the processing process, the stone becomes a diamond, acquiring a high price. The second role of the gem is material.
For some, diamonds are an investment option.
View this post on Instagram
Publication from Jewelry store "ALMAZ" (@almazz_66) June 5, 2019 at 10:50 PDT
A gem is not always ideal in color and transparency. There are cloudy samples; during jewelry processing, small fragments remain that cannot be used for inlaying jewelry. They are used in precision instruments:
- electrical engineering;
- radio electronics;
- glass cutters;
- power electronics;
- drilling rigs.
Graphite
Main areas of application:
- fire-resistant equipment;
- lubricants;
- pencil leads;
- neutron moderator in nuclear power;
- creation of artificial diamonds.
Diamond can only be used as a hard crystal, graphite as a greasy paste and a hard object.
In addition, watch the video:
Industrial Applications
Diamond is not scratched by any existing element on Earth. This remarkable property has become widespread in the field of national economy. Two allotropic states of one chemical element carbon - graphite and diamond, but have such different uses. Graphite, with the lowest hardness, is used as a dry lubricant in friction mechanisms, while diamond, with the highest Mohs hardness, is used as an abrasive material. Diamond-coated drill bits and grinding wheels are a small part of the materials processing production tools.
Diamond has also found its use in space exploration as a heat-removing material at extreme temperatures.
The Pioneer station, launched towards Venus in 1978, was covered with material made from diamond chips.
The widespread use of technical samples (artificially obtained) is known in radio electronics, optical instruments, and in the production of medical instruments. For technical needs, 500 million carats of artificial diamonds are produced, which is 100 tons annually.
Comparative characteristics of diamond and graphite
Characteristics Analysis:
- A comparison of crystal and graphite from the point of view of inorganic chemistry indicates that they are the same. Carbon is the basis of the composition.
- Comparison at the molecular level helps to understand the reason for this difference in properties. The arrangement of atoms and the bonds between them makes the same different.
- Physical properties differ radically, except for the ability to conduct heat.
- A comparison of the scope of application suggests that they are used based on the structure of the crystal lattice, and not on the chemical composition.
Comparison of the composition, structure, and use of two different substances shows how similar things can have polar characteristics and properties.
Do you think that when comparing other substances you can find similarities and differences at the same time? Share information with friends and acquaintances on social networks.
What are crystals and their diversity in nature
The origin of the word “crystal” is interesting. It sounds almost the same in all European languages. Many centuries ago, among the eternal snows of the Alps, on the territory of modern Switzerland, very beautiful, completely colorless crystals were found, very reminiscent of pure ice. Ancient naturalists called them “krystallos”, translated from Greek as ice. It was believed that ice, being in the mountains for a long time in severe frost, petrifies and loses the ability to melt. One of the most authoritative philosophers of antiquity, Aristotle, wrote: “Crystallos is born from water when it completely loses heat.” The Roman poet Claudian described the same thing in verse:
In the bitter alpine winter, the ice turns to stone.
The sun is then unable to melt such a stone.
A similar conclusion was made in ancient China and Japan - ice and rock crystal were designated by the same word. And even in the 19th century these images were often combined together.
Barely transparent ice, dimming over the lake,
The crystal covered the motionless jets.
A. S. Pushkin to Ovid
There is a great variety of crystals. They are different in color and transparency.
(presentation “variety of crystals”) And who hasn’t admired snowflakes, the variety of which is endless! Back in the 17th century, the famous astronomer Johannes Kepler wrote a treatise “On Hexagonal Snowflakes,” and three centuries later, albums were published containing thousands of photographs of enlarged snowflakes, none of them repeating the other. (presentation of “snowflakes”).
Fullerene
Fullerene , buckyball, or buckyball is a molecular compound that belongs to the class of allotropic forms of carbon and is a convex closed polyhedron composed of an even number of three coordinated carbon atoms.
The unique structure of fullerenes determines their unique physical and chemical properties. In combination with other substances, they make it possible to obtain materials with fundamentally new properties. Mining history:
The discovery of the fullerene resulted from experiments by Smalley and Croteau with an instrument that Smalley had invented to study molecules and atomic clusters. Kroteau was interested in Smalley's proposed laser evaporation technique. With its help, he intended to test his theory about the behavior of carbon in interstellar space. Croteau believed that carbon-rich red giants were capable of emitting complex carbon compounds that could be detected by radio telescopes.
Fullerene structure:
Atom bond Fullerene is a new allotropic form of carbon. Fullerene molecules consist of 60.70 atoms forming a sphere. Crystalline fullerenes are semiconductors. The variety of physicochemical and structural properties of compounds based on fullerenes allows us to talk about the chemistry of fullerenes as a new promising direction in organic chemistry.
Carbon atoms are located at the vertices of regular hexagons and pentagons that make up the surface of a sphere or ellipsoid. The most symmetrical and most fully studied member of the fullerene family is fullerene (C60), in which the carbon atoms form a truncated icosahedron consisting of 20 hexagons and 12 pentagons and resembling a soccer ball.
The next most common is the C70 fullerene, which differs from the C60 fullerene by the insertion of a belt of 10 carbon atoms into the equatorial region of C60, as a result of which the C70 molecule is elongated and resembles a rugby ball in shape. The so-called higher fullerenes, containing a larger number of carbon atoms (up to 400), are formed in much smaller quantities and often have a rather complex isomeric composition. Among the most studied higher fullerenes are Cn, n=74, 76, 78, 80, 82 and 84.
Boron nitrile
This substance appeared relatively recently: it was synthesized in laboratory conditions in 1957, and turned out to be much harder than diamond. At the same time, it surpasses it in a number of other properties. For example, when exposed to ultra-high temperatures, the substance does not dissolve in metals, so it can be used for processing steel. A layer of carbon-boron nitrile is applied to the tool as a cutting edge for machining all kinds of parts used in aircraft and spacecraft.
Nature is amazing and many more incredible discoveries await us. Diamond is far from the hardest substance in the world. True, it is not easy to compete with other minerals in terms of beauty and attractiveness. Although it cannot be ruled out that sooner or later wedding rings with fullerite or lonsdaleite will appear on sale.
The concept of allotropy
The concept of “Allotropy” has ancient Greek roots: αλλος - other, τροπος - property. Allotropy is the existence of two or more simple substances of the same chemical element. The concept of allotropy was introduced into science by J. Berzelius in 1841 to designate different forms of existence of elements.
The phenomenon of allotropy implies the possibility of creating a certain amount of different substances from the same element. For example, oxygen and ozone contain only oxygen in their composition. The question of how this is even possible has interested many people for a long period of time. Today, scientists can easily explain all the features of this process.
Not all elements are capable of forming several different simple substances. This ability directly depends on the structure of the molecules. Most often, this phenomenon is observed in elements that have variable oxidation states.
This applies to groups such as:
- non-metals;
- semimetals;
- noble gases;
- halogens.
The causes of allotropy can be of several types. The most likely of them, scientists include the following factors:
- Different numbers of atoms required to form one molecule.
- A different order of conjugation of atoms into one molecule.
- Parallels between electron spins.
- A type of crystal lattice.
In order to clearly understand how the phenomenon of allotropy can exist, it is necessary to consider several of the most remarkable examples that are widely found in nature.
Examples of allotropy:
When metals are heated during the transformation process, heat is absorbed, and the change in lattice structure occurs at the same temperature. Many metals are subject to allotropic modifications, for example, titanium, iron, tin, etc. When heated to +1390 ºC, iron is characterized by a face-centered lattice. An increase in temperature to +1540 ºС leads to a restructuring to a centered cubic structure.
Various substances
- Diamond. The mineral is of high value and, after cutting, is used in jewelry. So what is the secret of the popularity of this stone? Carbon atoms form the basis of the entire lattice. There is a strong covalent bond between the atoms of the mineral. The crystal lattice of diamond is characterized by a dense content of atoms in the form of a cube. In other words, carbon atoms are considered nodes, and the peculiar faces of the cube are strong covalent bonds. This mineral is considered the most durable on the planet, and it is unknown how many of these unique cubes include a solid diamond.
- Graphite. Carbon can also be in another crystalline modification. The atomic lattice of this element includes only carbon atoms and has a layered structure. In graphite, each atom is bonded by three carbon atoms. Because of this, it has a metallic luster and high thermal conductivity.
- The crystal lattice of iodine is of a molecular type. Atoms of molecules are connected by covalent bonds, but the molecules of a chemical element have weak attractive forces. This characterizes iodine in that it has low hardness and a low melting point.
- Sodium. Representative of a metal crystal lattice. Electrons move between cations located at lattice sites. By joining cations, they neutralize their charge, in turn, neutral atoms release some electrons, transforming into cations. This type of crystal lattice gives the metal plasticity, electrical and thermal conductivity.
- Dry ice. Or carbon monoxide in solidified form. It has a molecular crystal lattice in the shape of a cube. Molecules are held together by weak bonds. infusion, read our article.
This is interesting: how to determine valency using the periodic table?
Theory of the origin of diamonds
The term diamond comes from the Greek word “adamas”, which translates as unsurpassed. Today there is no consensus on the origin of diamonds.
The most popular hypothesis is that the mineral was formed during the cooling of silicates in the mantle of the earth’s crust. And it appeared on the surface of the earth as a result of a series of very powerful underground explosions.
Wurtzite boron nitride
The crystal lattice of this substance is a special form called wurtzite. This is what makes the substance so hard. If you apply a load to the crystal, the atoms in the crystal lattice will be redistributed in a special way, causing the substance to become even harder.
That is, the greater the load, the harder boron nitride becomes! This property makes it similar to lonsdaleite, another “competitor” of diamond, which forms at the bottom of craters left by graphite meteorites. Until now, it has not been possible to establish exactly why the hardness of the mineral changes when exposed to loads.
Unfortunately, the question remains open, since experimenting with this substance is quite difficult, since it is not easy to synthesize in the laboratory.
Graphite
Graphite is a mineral from the class of native elements, one of the allotropic modifications of carbon. The structure is layered. The layers of the crystal lattice can be differently positioned relative to each other, forming a number of polytypes, with symmetry from hexagonal system (dihexagonal-dipyramidal) to trigonal (ditrigonal-scalenohedral).
The layers are slightly wavy, almost flat, and consist of hexagonal layers of carbon atoms.
Mining history:
In the 60s of the 16th century in England. Local shepherds, who found deposits of a strange black-shiny material, at first mistook it for lead, but, realizing that they couldn’t cast bullets from it, they began to break off pieces of black stone and mark their sheep with it. The new material soon attracted the attention of artists and merchants, who quickly established trade in thin plates and pieces of graphite on the English streets. Of course, it was very inconvenient to use - your hands got dirty! I had to wrap the graphite with rope, paper, or even simply press it between boards. This is how the first pencils in a wooden case appeared.
Graphite structure:
Physical properties in graphite vary greatly in directions - perpendicular and parallel to the layers of carbon atoms.
When heated without air access, graphite does not undergo any changes up to 3700°C. At the specified temperature, it sublimes without melting.
Artificial graphite is produced from the best grades of coal at 3000°C in electric furnaces without air access.
Graphite is thermodynamically stable over a wide range of temperatures and pressures, so it is accepted as the standard state of carbon. The density of graphite is 2.265 g/cm3.
There are two known forms of graphite: alpha graphite (has a hexagonal structure and crystal lattice) and beta graphite (has a rhombohedral structure and crystal lattice). In α-graphite, half of the atoms of each layer are located above and below the centers of the hexagon, and in β-graphite, every fourth layer repeats the first.
Alpha graphite can be converted to beta form by mechanical processing. The beta form transforms into the alpha form when graphite is heated above 1300 °C.
Lonsdaleite
This mineral is very similar to diamond in its molecular structure. It is even called hexonal diamond. Lonsdaleite is also one of the carbon modifications.
However, if this substance is contaminated with various impurities, it cannot boast of particular hardness. But when refined, it is much harder than diamond and can easily scratch it. Pure lonsdaleite is 58% stronger than diamond, and when a load is applied to it, its strength only increases. By the way, the mechanism of this process still remains a mystery to scientists.
The story of its discovery is very interesting. For the first time, traces of the substance were found at the bottom of craters left after the fall of meteorites. These meteorites apparently consisted mainly of graphite. Due to the high temperature, the graphite turned into lonsdaleite. The mineral was found in Russia at the site of the Tunguska meteorite, and also in America in the Devil's Crater. Due to this, Lonsdaleite is also called the cosmic diamond.
The mineral received its name in honor of British mineralogist Kathleen Lonsdale. The idea to give it just such a name was proposed by another mineralogist named Clifford Frondel. He explained this idea by saying that a new form of diamond in nature is as rare as a female scientist. Of course, this is not so relevant these days. In the 1960s, the situation in science was such that it was difficult for women to achieve great scientific heights.