Hello, our dear readers! Have you ever wondered what diamond and graphite might have in common? It would seem that a diamond is what expensive jewelry is made from, pleasing the eye of even the most refined taste. Hard, tough and virtually indestructible. And graphite, the main element for making pencils, is very fragile and breaks easily. Remember how often your stylus broke?
However, both minerals are related to each other. Moreover, recreating special conditions makes it possible to carry out the process of transformation from graphite to diamond, and vice versa.
Reading the article will allow you to find out what properties the minerals presented in the article have, how they appeared on Earth in the first place, and where you need to go in order to mine diamonds. Or, if you’re less lucky, graphite, and also, is it possible to make diamonds and graphite at home?
We wish you pleasant reading!
Properties of diamond and graphite
Briefly about allotropic carbon and carbyne. In the periodic table of Mendeleev, this type of non-metal is located at number 6. The valence state of carbon radically affects the properties of the substance in which it is present.
Despite the fact that diamond differs from graphite in all characteristics, both minerals are built from free carbon.
Chemical properties
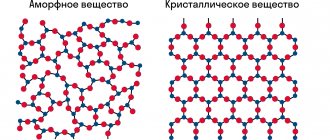
The structure of a diamond crystal is spatial. Diamond and graphite are tetrahedrons firmly connected to each other, inside which the atoms with a covalent bond are spaced equally apart from each other. The carbon share is approaching 99.8%. Minor impurities affect the “purity” and shade of the nugget. It is known to be chemically resistant to acids and alkalis.
It is important to remember that at a high combustion temperature of 800 to 1000 degrees in air, diamond molecules turn into a pile of graphite.
The essence of the graphite crystal lattice consists of layers. The individual layers look like interconnected hexagons, similar to a honeycomb. The arrangement of layers relative to each other is not structured and may vary in nuggets. Atoms are tightly bound only within one layer. Adjacent layers do not have rigid bonds between atoms. Contain various inclusions. Graphite does not dissolve in acids. At high temperatures it burns to an amorphous gas, interacting with oxygen. Alkali metals and salts can form “inclusion compounds” with it.
Physical properties
The difference in the structure of diamond and graphite also determines different physical properties:
- Hardness. Diamond is the hardest and densest mineral created by nature. It has a minimum compression ratio. Graphite has a soft structure and feels greasy to the touch. Despite the different density values, both are fragile and will crumble when dropped or hit.
- Transparency. Opaque, gray or dark gray graphite absorbs light. When rubbed, it peels off and leaves dark marks on the surface. Metal inclusions give the nugget shine. The structure of the crystals in diamond gives transparency. Natural gems are not always completely transparent and colorless. Some have a colored tint. Turbid crystals are valued lower.
- Thermal conductivity. Diamond has the highest index compared to other solids. An excellent semiconductor capable of operating at high temperatures. Graphite has extremely low thermal conductivity.
- Electrical conductivity. If you measure this parameter along graphite layers, the result will be quite high, close to metal. Across the planes it is hundreds of times smaller, and the highest is for recrystallized graphite. Diamond does not conduct electricity; it is a dielectric.
Graphene
Instead of compressing and heating graphite, we, following Andrei Geim and Konstantin Novoselov, will glue a piece of tape to the graphite crystal. Then peel it off - a thin layer of graphite will remain on the tape. Let's repeat this operation again - apply the tape to a thin layer and peel it off again. The layer will become even thinner. By repeating the procedure a few more times, we obtain graphene, the material for which the aforementioned British physicists received the Nobel Prize in 2010.
Graphene is a flat monolayer of carbon atoms, completely identical to the atomic layers of graphite. Its popularity is due to the unusual behavior of the electrons in it. They move as if they have no mass at all. In reality, of course, the mass of electrons remains the same as in any substance. The carbon atoms of the graphene frame are to blame for everything, attracting charged particles and forming a special periodic field.
Graphene-based device. In the background of the photo are gold contacts, above them is graphene, above is a thin layer of polymethyl methacrylate
Engineering at Cambridge / flickr.com
Share
The consequence of this behavior is the greater mobility of electrons - they move in graphene much faster than in silicon. For this reason, many scientists hope that graphene will become the basis of the electronics of the future.
Interestingly, graphene has carbon cousins - pentagraphene and phagraphene. The first of them consists of slightly distorted pentagonal sections and, unlike graphene, does not conduct electricity well. Phagraphene consists of pentagonal, hexagonal and heptagonal sections. If the properties of graphene are the same in all directions, then phagraphene will have a pronounced anisotropy of properties. Both of these materials have been theoretically predicted, but do not yet exist in reality.
A fragment of a silicon single crystal (in the foreground) on a vertical array of carbon nanotubes
zeiss.com
Differences between graphite and diamond
When comparing the main characteristics, significant differences are found. Some qualitative parameters are directly opposite.
On the ten-point Mohs scale, diamond has a hardness of 10, and graphite has a hardness of 1.
Light can easily pass through the gem crystal. Using this property, the gem is cut in a special way and a diamond is obtained. The rays reflected from the edges play with all the colors of the rainbow. Graphite is opaque and absorbs light.
Graphite has magnetic susceptibility.
The thermal conductivity of diamond is from 900 to 2300, and that of graphite is not higher than 350 W/(m K). The first is a dielectric, and the electrical conductivity of the second approaches those of metals.
Interestingly, when heated, diamond is preserved up to 720 degrees Celsius, and graphite - up to 3700. When heated to 700 degrees Kelvin, graphite contracts, and with a further increase in temperature, it expands.
Carbin
When talking about elongated structures of carbon atoms, one cannot fail to mention carbines. These are linear chains, which, according to theorists, may turn out to be the strongest material possible (we are talking about specific strength). For example, the Young's modulus for carbyne is estimated at 10 giganewtons per kilogram. For steel this figure is 400 times less, for graphene it is at least two times less.
A thin thread stretching to the iron particle below - carbine
Wikimedia Commons
Share
Carbynes come in two types, depending on how the bonds between the carbon atoms are arranged. If all the bonds in the chain are the same, then we are talking about cumulenes, but if the bonds alternate (single-triple-single-triple and so on), then we are talking about polyines. Physicists have shown that a carbyne thread can be “switched” between these two types by deformation - when stretched, cumulene turns into polyyne. Interestingly, this radically changes the electrical properties of carbyne. While polyyne conducts electric current, cumulene is a dielectric.
The main difficulty in studying carbines is that they are very difficult to synthesize. These are chemically active substances that are also easily oxidized. To date, chains of only six thousand atoms in length have been obtained. To achieve this, chemists had to grow carbyne inside a carbon nanotube. In addition, the synthesis of carbyne will help break the record for the size of the gate in a transistor - it can be reduced to one atom.
How to get diamond from graphite
Natural resources are finite. The industry is developing rapidly. Diamond and graphite are used to increase the demand for materials with these properties. If the original minerals consist of the same element, is it possible to obtain diamond from graphite?
Under natural conditions, diamond nuggets were formed under high pressure and sudden cooling. In the laboratory, they simulated an explosion, obtaining the transition of graphite into many small diamond crystals. After numerous experiments in which graphite was heated to different temperatures under different combinations of pressure, scientists developed a method for producing artificial diamonds.
The process involves the transformation of covalent bonds. The crystal lattice of graphite is destroyed under the influence of high temperature and pressure. When using a catalyst, a new crystal grows - a diamond. The technology is complex. Everything happens in a durable chamber. The press creates a high pressure of 1010 Pa. A "solution" of graphite mixed with the agent is heated to almost 3000 degrees using an electric current. After diamond synthesis, the temperature and pressure are gradually reduced.
The crystals are obtained with the required properties, but are opaque. For mass industrial use, “purity” is not a decisive indicator. Since the middle of the last century, artificial diamond has replaced its natural counterpart in technical products. Growing large and pure crystals turned out to be expensive and unprofitable.
"Explosion" or "shock wave" is not widely used. Work on studying this method continues. Transparent crystals obtained in this way are similar to natural samples, but their size is small.
Carbon nanotubes
Imagine that you rolled a small piece of graphene sheet into a tube and glued its edges together. The result is a hollow structure consisting of the same hexagons of carbon atoms as graphene and graphite - a carbon nanotube. This material is in many ways related to graphene - it has high mechanical strength (it was once proposed to build an elevator into space from carbon nanotubes) and high electron mobility.
However, there is one unusual feature. The graphene sheet can be rolled parallel to an imaginary edge (the side of one of the hexagons), or at an angle. It turns out that how we twist a carbon nanotube will greatly influence its electronic properties, namely whether it will be more like a semiconductor with a bandgap or more like a metal.
Multi-walled carbon nanotube
Wikimedia commons
Share
It is not known for certain when carbon nanotubes were first observed. In the 1950s to 1980s, various groups of researchers involved in the catalysis of reactions involving hydrocarbons (for example, methane pyrolysis) paid attention to elongated structures in the soot covering the catalyst. Now, in order to synthesize carbon nanotubes of only a specific type (specific chirality), chemists propose using special seeds. These are small molecules in the form of rings, which in turn consist of hexagonal benzene rings. You can read about the work on their synthesis, for example, here.
Like graphene, carbon nanotubes have many applications in microelectronics. The first transistors based on nanotubes have already been created, surpassing traditional silicon devices in their properties. In addition, nanotubes formed the basis of the transistor with the smallest gate in the world.
Applications of diamond and graphite
Both of these substances are used by humans both in industry and in everyday life. The characteristic properties determine the scope of application.
There are jewelry and technical diamonds. No more than 22% of gems are used in jewelry. For this purpose, the best, usually natural, stones are selected. They are cut taking into account the structure. All kinds of jewelry are created from the resulting diamonds. Synthetic stones are also used. Products made from them look beautiful, but there are differences. The presence of the smallest inclusions, the shade of the edges and the influence of the magnet will give away an artificial diamond.
Second-class materials are used in technical products. Whole crystals, fragments and even “dust” from grinding the mineral go to a just cause. Diamonds of the appropriate type and size are selected for bearings, drill tips, and drills. Raw crystals with a sharp tip are used in electronics. Small, defective specimens and fragments are crushed into diamond powder. The crumbs are sprayed onto the edges and planes of cutting and sharpening discs and grinding wheels.
There is an assertion that when drilling wells in dense rocks, the use of a diamond bit saves time, resources and reduces overall costs. Diamond-coated tools are designed for grinding surfaces. Diamond has long been used for cutting glass, metal and other materials. The watch industry cannot do without these stones. More than a thousand industrial products contain various types of gems.
The scope of graphite is also extensive. In everyday life, the mineral is used in the manufacture of pencil leads. Graphite is the basis of solid lubricants and is included in plastics, paints, and electrically conductive adhesives. In electrical machines it is present in brushes, current collectors, rheostats and wherever a movable electrical contact is needed. In metallurgy, raw graphite and carbon black are used in the smelting of steel and aluminum.
In nuclear energy, graphite rods, which moderate neutrons in nuclear reactors, are the most important element of the reactor. For military and space purposes it is used to protect the rocket body from overheating.
Mineral deposits
Diamonds originate at a depth of 100 km and at a temperature of 1300 degrees. Kimberlite magma, which forms kimberlite pipes, comes into action through explosions. It is these pipes that represent the primary diamond deposits. The first such pipe was discovered in the African province of Kimberley, where its name came from.
The most famous deposits are located in India, Russia and South Africa. Primary deposits account for 80% of all mined diamonds.
To find a diamond in nature, X-rays are used. Most of the stones that are found are unsuitable for jewelry production, as they have a significant number of defects, including cracks, inclusions, foreign fluorescent shades, and so on. Therefore, their use is technical. Such stones are divided into three categories:
- board - stones with a zonal structure;
- ballas - stones that are round or pear-shaped;
- carbonado - black diamond.
Diamonds that are large in size and have outstanding characteristics tend to get their name. In addition, the high cost of the stone makes it desirable for many, which guarantees a “bloody history.”
Graphite is formed by alteration of sedimentary rocks. In Mexico and Madagascar you can find low quality graphite ore. The most famous deposits are in Krasnodar and Ukraine.
Q-carbon
Among the recently discovered forms of carbon is the so-called Q-carbon. It was first synthesized by American materials scientists from the University of North Carolina in 2015. Scientists irradiated amorphous carbon using a powerful laser, locally heating the material to 4000 degrees Celsius. As a result, approximately a quarter of all carbon atoms in the substance adopted sp2 hybridization, that is, the same electronic state as in graphite. The remaining Q-carbon atoms retained the hybridization characteristic of diamond.
Q-carbon
ncsu.edu
Share
Unlike diamond, graphite and other forms of carbon, Q-carbon turns out to be ferromagnetic, such as magnetite or iron. At the same time, its Curie temperature was about 220 degrees Celsius - only with such heating did the material lose its magnetic properties. And by doping Q-carbon with boron, physicists obtained another carbon superconductor, with a transition temperature of about 58 kelvin.
Fullerenes
Although the hexagon is one of the most stable configurations that carbon atoms can form, there is a whole class of compact objects where the regular carbon pentagon occurs. These objects are called fullerenes.
In 1985, Harold Kroteau, Robert Curl, and Richard Smalley studied carbon vapor and how carbon atoms clump together when cooled. It turned out that there are two classes of objects in the gas phase. The first is clusters consisting of 2–25 atoms: chains, rings and other simple structures. The second is clusters consisting of 40–150 atoms, which have not been observed before. Over the next five years, chemists were able to prove that this second class consists of hollow frameworks of carbon atoms, the most stable of which consists of 60 atoms and is shaped like a soccer ball. C60, or buckminsterfullerene, consisted of twenty hexagonal sections and 12 pentagonal sections, bonded together to form a sphere.
The discovery of fullerenes aroused great interest among chemists. Subsequently, an unusual class of endofullerenes was synthesized - fullerenes in the cavity of which there was some foreign atom or small molecule. For example, just a year ago a hydrofluoric acid molecule was placed into a fullerene for the first time, which made it possible to very accurately determine its electronic properties.
Fullerites - crystals of fullerenes
Wikimedia Commons
Share
In 1991, it turned out that fullerides—fullerene crystals in which some of the cavities between adjacent polyhedra are occupied by metals—are molecular superconductors with a record high transition temperature for this class, namely 18 kelvin (for K3C60). Later, fullerides with an even higher transition temperature were found - 33 kelvin, Cs2RbC60. Such properties turned out to be directly related to the electronic structure of the substance.
Table
The compact table provides examples of the differences between graphite and diamond. The differences between these carbon-based minerals perfectly illustrate how different allotropes of the same chemical element can be.
| Graphite | Diamond | |
| What does it consist of? | Carbon has a layered structure | Carbon has a cubic crystal lattice |
| Distribution in nature | Widely distributed. In Russia - on the Kola Peninsula, in the Urals and Siberia | Distributed relatively widely, but due to the difficulties of mining and processing, diamond products are expensive |
| Application | Widely used in a wide variety of industries: in the production of pencils, various lubricants, in the nuclear, space industries, metallurgy and some others | Jewelry industry, production of working parts for cutting and abrasive tools, most recently in the radio-electronic industry |