The melting point of diamond is one of the characteristics of jewelry that has not yet been fully studied. The stone has unique properties that are valued not only in jewelry, but also in industry. And the melting point was no exception to the rule.
Some mineralogists and researchers explain such strange characteristics of diamond by its cosmic origin. That is, it is assumed that the material fell on the planet after the fall of a large number of meteorites and remained in the bowels of the earth.
How were diamonds formed in the depths of the Earth?
According to scientists, diamonds could have appeared during the formation of the planet's core as a result of the impact of enormous pressure on the molten magma. Precious stones advanced to the surface areas of the earth's crust due to gas formation processes in deep rocks. As a result, so-called diamond pipes were formed, which are voids in rocky soil with large mineral deposits.
Variety of colors
Most people mistakenly believe that the variety of diamond is limited only to transparent, colorless crystals. In fact, there are quite a lot of different color variations, which are sometimes priced much more expensive than the classic ones.
Jpg» alt=»» width=»80″ height=»83″> Yellow diamond is quite common. The mineral received this color due to nitrogen atoms that penetrated its crystal lattice. The more saturated the color, the more expensive the sample will cost. There are also darker variations that are found in Australia. There you can find both a cognac diamond and a red diamond.
Jpg» alt=»» width=»80″ height=»83″> Blue diamond is a real rarity. It may be a natural variety that gets its color from the presence of atoms of a chemical such as boron. A blue diamond can also be obtained by refining the mineral.
Jpg» alt=»» width=»80″ height=»83″> But the blue diamond (its large specimens) is so rare that only holders of luxury collections can afford it. A more common diamond is a diamond whose color turns blue as a result of heat and pressure.
Every jeweler would not mind adding a green diamond to their collection, which gets its color due to natural radiation. Red diamonds are even rarer. They, like pink diamonds, are mined in Australian deposits.
The types of diamonds don't end there. There are even black and white diamonds.
Material properties
Before finding out what the melting point of diamond is, let's look at the properties of the mineral:
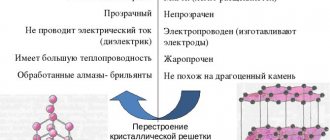
- Diamonds have the highest hardness of any existing fossil. For this reason, no material is capable of destroying the structure of a diamond or scratching its surface. He himself can damage any physical object.
- Diamond is a highly effective insulator. It is resistant to acids and other aggressive chemical environments.
- Diamond has the highest thermal conductivity of all solid minerals. The gem can be held in the palm of your hand as long as you like. At the same time, its temperature will remain unchanged.
- Diamond has a unique luminescence. Light rays of any origin, when passing through a mineral, make it glow brightly and shimmer with all the colors of the rainbow.
What is the Mohs Hardness Scale for Metals and Minerals
The Mohs scale is a measurement table that allows you to find out the hardness of a mineral and stone material. Such a scale appeared in the 19th century, and the name is identical to the name of the creator. After developing and conducting a number of studies, the measurement indicator became very popular due to its maximum accuracy and efficiency in the context of splitting production activities.
Determining the index of metals and minerals on the Mohs scale allows you to select the correct material processing technology to create comfortable operating conditions. And thanks to accurate indicators, accurate information is provided in terms of whether a diamond can be melted or whether it is better to break it with a hammer, and whether this is even possible. The technical properties of each stone material are ideally described on the scale of a renowned geologist.
Diamond Melting Conditions
In 2010, in the course of experiments, physicists from the laboratory of the University of California, located in Berkeley, determined the level of temperature exposure on diamond, which leads to its melting. Scientists have found that it is impossible to convert the material into liquid form under normal conditions, regardless of the level of heating. This goal can be achieved only by exposing the diamond not only to temperature, but also to high pressure. It is necessary to increase the pressure so that the mineral does not turn into graphite. Thus, the transition of diamond into liquid form is an extremely difficult process.
APPLICATION
Good crystals are cut and used in jewelry. About 15% of mined diamonds are considered jewelry, another 45% are considered near-jewelry, that is, inferior to jewelry in size, color or clarity. Currently, global diamond production is about 130 million carats per year. Diamond
(from the French brillant - brilliant), - a diamond, which through mechanical processing (cutting) has been given a special shape, a brilliant cut, which maximizes the optical properties of the stone such as brilliance and color dispersion. Very small diamonds and fragments unsuitable for cutting are as an abrasive for the manufacture of diamond tools necessary for processing hard materials and cutting the diamonds themselves.
The cryptocrystalline variety of diamond, black or dark gray in color, forming dense or porous aggregates, is called Carbonado
, has a higher abrasion resistance than diamond crystals and is therefore especially valued in industry.
Small crystals are also grown artificially in large quantities. Synthetic diamonds are obtained from various carbon-containing substances, mainly from graphite, in special. apparatuses at 1200-1600°C and pressures of 4.5-8.0 GPa in the presence of Fe, Co, Cr, Mn or their alloys. They are suitable for technical use only.
Diamond - C
| Molecular weight | 12.01 g/mol |
| origin of name | From Greek, adamas, meaning "invincible" or "solid". |
| IMA status | valid, first described before 1959 (before IMA) |
What is the melting point and boiling point of diamond?
According to data obtained during the study of the properties of the material, its melting in air space under high pressure occurs when heated to 850-1000 oC.
Diamond can be brought to a boil by exposing it to a temperature of 1800 to 2000 °C in a vacuum. In both cases, when cooled, the mineral transforms into graphite. To determine the melting point of diamond, scientists conducted experiments using a small natural mineral, the mass of which was 1/10 of a carat. The boiling of the surfaces of the material occurred under the influence of a shock wave created by short-term laser pulses.
Researchers were able to establish what the melting point of diamond is (in degrees) only by creating a pressure that was 40 million times higher than the normal atmospheric pressure at sea level. When the pressure dropped to 11 million atmospheres, solid particles began to form on the surface of the boiling mineral, which did not sink, but floated like ice in water.
MORPHOLOGY
Diamond morphology is very diverse. It occurs both in the form of single crystals and in the form of polycrystalline intergrowths (“board”, “ballas”, “carbonado”). Diamonds from kimberlite deposits have only one common flat-faceted shape - the octahedron. At the same time, diamonds with characteristic curved shapes are common in all deposits - rhombic dodecahedroids (crystals similar to a rhombic dodecahedron, but with rounded edges), and cuboids (crystals with a curved shape). As experimental studies and the study of natural samples have shown, in most cases, dodecahedroid-shaped crystals arise as a result of the dissolution of diamonds by kimberlite melt. Cuboids are formed as a result of the specific fibrous growth of diamonds according to the normal growth mechanism.
Cullinan diamond broken into 9 parts
Synthetic crystals grown at high pressures and temperatures often have cube faces and this is one of their characteristic differences from natural crystals. When grown in metastable conditions, diamond easily crystallizes in the form of films and columnar aggregates. The sizes of crystals vary from microscopic to very large, the mass of the largest diamond “Cullinan”, found in 1905. in South Africa 3106 carats (0.621 kg). Several months were spent studying the huge diamond and in 1908 it was split into 9 large parts. Diamonds weighing more than 15 carats are rare, and those weighing over a hundred carats are unique and are considered rarities. Such stones are very rare and often receive their own names, world fame and their special place in history.
Where are diamonds found in the earth's crust?
These minerals are extremely rare. However, industrial deposits today are being developed on almost all continents of the globe. The only exception is Antarctica.
Until the mid-19th century, it was believed that minerals formed in river sediments. Later, the first diamond-bearing cavities were discovered in rocky mountain soil at a depth of several hundred meters.
According to scientists, the age of some diamonds ranges from 100 million to 2.5 billion years. Researchers managed to obtain “older” minerals of unearthly origin. The latter were brought to the planet along with meteorites that formed in outer space even before the formation of the Solar System.
Place of Birth
Rough Diamond
Industrial diamond deposits are scattered throughout the planet, with the only exception being Antarctica.
In percentage terms, the production volume was distributed as follows:
- First place - Russian, which also develops mines in Botswana, Zimbabwe and Angola - 28%.
- The second is the South African corporation De Beers, which produces in Tanzania, Namibia, South Africa and Botswana - 20%.
- Third – Australian-British miner Rio Tinto – 13%.
- Fourth – Canadian company Dominion Diamond – 6%.
- Fifth – South African company Petra Diamonds – 3%.
- The remaining 30% of production comes from all other diamond miners.
The very first deposits are the mines of India, which by the end of the 20th century were practically depleted. In 1727, the richest deposits of diamonds were discovered in Brazil, in 1867 - in South Africa.
This is interesting!
In Russia, the first diamond was discovered in July 1829. This event took place in the Perm Province (Ural) at the Krestovozdvizhensky gold mine. A stone weighing half a carat was found by 14-year-old serf Pavel Popov, for which he received his freedom. A little later, the teenager brought members of Baron Humboldt’s expedition to the same place, who discovered several more small pebbles. At the moment, the treasured place is called “The Diamond Key”. Today, the richest Russian deposits are located in Yakutia. At the end of 2019, it was there that the first Matryoshka diamond in the history of world mining was found - a gem within which another smaller pebble moves freely.
Do diamonds exist in molten form in natural conditions?
The melting point of diamond is so high that the mineral can no longer exist in boiling form on Earth.
However, what about space objects? According to scientists, the melting point of diamond is still maintained in the depths of planets such as Neptune and Uranus. It is noteworthy that the latter are 10% formed from carbon, which is the structural basis of this mineral. According to many scientists, on the above planets there are entire oceans of diamonds in liquid, boiling form. This hypothesis explains why the magnetic field of these celestial bodies behaves so strangely. After all, Neptune and Uranus are the only planets in the solar system whose geographic poles do not have a clear position and are literally separated in space. To confirm an interesting hypothesis, all that remains is to simulate similar conditions on Earth experimentally. However, such a solution currently remains extremely expensive and time-consuming. Therefore, it is not yet possible to determine for sure whether there really are entire oceans of molten diamonds on nearby planets.
Compatibility with other stones
Diamond is the real king of gems - dazzling and powerful, luxurious and expensive. It does not tolerate proximity to semi-precious and ornamental stones. The best company for him is jewelry of approximately equal value, but not all. Stones belonging to the element of water are excluded - these are emerald, chrysoberyl, aquamarine, opal, selenite, coral and other gems. The rule does not apply only to pearls.
The best combination is between diamonds and rubies and pyropes, as they complement each other both aesthetically and energetically.
Blue Diamond
The combination of water-air sapphire and fire diamond is ambiguous; the consequences of such a combination are difficult to predict.
[edit] “Granite gravy”
In addition to the memetic “Artisan's Handbook”, the recipe from which was mentioned at the beginning of the article, there are several other pieces of evidence from historical sources that can be interpreted as “proof” of working with liquid granite:
Publication by A. A. Iovsky
“On the Importance of Chemical Research...” 1827, where the following words are quoted:
| ... the production of granite through melting, the transformation of chalk into marble through the placement of onago principles under the action of heat, ... |
The phrase is taken out of context. The quote lists the latest achievements of chemistry as a young science. In this case, the ancient chemists learned that granite was formed from magmatic melt, and not what the lithics thought.
In the publication of priest Serafimov “Description of St. Isaac’s Cathedral in St. Petersburg...” in 1865, when listing the types and costs of work on the construction of the foundation, the item is mentioned: “For cutting and pouring granite.”
Expression of pouring stone, granite
is an obsolete construction term, the decoding of which is given in the “Journal of Communications and Public Buildings” of 1860:
| Laying granite cladding (pouring stones ) was carried out as follows... |
Step-by-step instruction
The crystal lattice of a diamond and its properties
In artisanal conditions, small bronze items, for example, decorative elements, are mainly made. More complex parts require high-precision casting, the technology of which is very difficult to implement without a room specially adapted for these purposes, as well as special equipment. In some cases, it is necessary to resort to finishing the casting to the desired state using at-home editing, such as removing excess molten material by hand, grinding and polishing the product.
Before you start melting metal, you need to prepare the room and acquire the necessary tools and equipment. The main requirement for the room is the presence of good exhaust ventilation, as well as a floor made of non-combustible materials such as concrete, cement or brick. When making small products, these requirements are quite easy to comply with, otherwise you will have to use the garage.
Preparing tools
A novice foundry worker should purchase or make the following tools.
- A refractory crucible made of a refractory material (such as cast iron or steel) is a special vessel with a spout into which pieces of metal to be melted are placed.
- Devices for removing the crucible from the furnace that minimize the risk of getting burned are special hooks and tongs.
- A mold for pouring molten metal, which is made using a flask and a model.
- The flask itself is two boxes that hold a casting mold filled with molding sand.
- A welder's suit or simply a very thick apron and mittens, the purpose of which is to protect a person from flying sparks and splashes of molten metal.
Once you are convinced that all of the above is present, you can proceed directly to melting bronze.
- Preheat the oven by setting the temperature using the regulator. The temperature depends on the chemical composition of the bronze, as we discussed above. For example, for aluminum bronze this temperature will be 1040-1084 degrees Celsius.
- Next, be sure to warm up the mold; this is done so that the molten metal does not freeze when it enters a cold container. The mold is placed in the oven when it warms up to a temperature of 600 degrees, after which the thermostat is set to 900 degrees. When the temperature inside the oven rises to 900 degrees, leave the mold to warm up for 3-4 hours, after which it is carefully removed using special devices and cooled to 500 degrees Celsius.
- Place a crucible with pieces of bronze intended for melting inside a furnace heated to the desired melting temperature and bring until the metal is completely melted. After this, leave the crucible to overheat for another 5 minutes to achieve better metal fluidity and better casting quality.
- Remove the crucible from the furnace or forge using hooks and tongs and begin pouring it into the mold.
Let's look at how to properly make a mold to obtain a high-quality product. In foundries, such a mold is made using a flask into which a mixture consisting of clay, sand and coal powder is poured. The flask consists of two halves, each of which is a box into which the molding sand will be poured.
- First, take the first box and begin to fill it with the mixture, filling it halfway, and place the model inside the box.
- Next, they continue to pour bulk material until the box is filled to the very top. During work, it is necessary to constantly level and compact the molding mixture.
- Place a second box on top and continue pouring a mixture of clay, sand and coal powder.
- In the second box it is necessary to provide sprues - holes for pouring molten bronze into the mold.
- When both boxes are filled to the top, separate them using a sharp object. One half of the model is in one box, the other half is in another.
- Carefully take out the model, connect both boxes again - the resulting void inside is the mold for filling.
Pouring into a mold
The molten metal is poured in a thin stream from the crucible into the mold, making sure that the stream flows continuously. If the part being cast has a complex shape, you need to use a special centrifuge, which, using centrifugal force, will help the melt to be correctly distributed inside the mold, completely filling it.
Contributions of Antoine Lavoisier to the study of crystal
The French physicist Antoine Lavoisier made a great contribution to the study of the mineral. He proved that diamonds burn in the presence of air. For his experiment he:
- placed the stone in a glass vessel;
- filled it with oxygen;
- corked.
Using a lens, he heated the diamonds, causing them to burn completely with a faint blue flame. But no ash was found in the flask. Having examined the air in the flask, he found out that carbon dioxide had appeared in it.
It is interesting that Lavoisier did not try to prove with his experiments that diamond can be burned - this happened by accident. The essence of his experiments was to refute the phlogiston theory.
Conducting experiments on burning substances in sealed capsules, Lavoisier could not attract the attention of the “scientific community” to them. To remedy this, he declared that he would burn a piece of diamond. This move proved the effectiveness of his work and revealed to the world one of the mysteries of the diamond.
History of experiments
In the 17th century in England, Boyle managed to burn a diamond by shining a sunbeam on it through a lens. However, in France, experience with calcination of diamonds in a melting vessel did not produce any results. The French jeweler who conducted the experiment found only a thin layer of dark plaque on the stones. At the end of the 17th century, Italian scientists Averani and Tardgioni, while trying to fuse two diamonds together, were able to establish the temperature at which a diamond burns - from 720 to 1000 ° C.
Diamond does not melt due to its strong crystal lattice structure. All attempts to melt the mineral ended with it burning.
The great French physicist Antoine Lavoisier went further, deciding to place diamonds in a sealed glass vessel and filling it with oxygen. Using a large lens, he heated the stones and they completely burned. Having studied the composition of the air, they found that in addition to oxygen, it contains carbon dioxide, which is a compound of oxygen and carbon. Thus, the answer was received: diamonds burn, but only with access to oxygen, i.e. on open air. When burned, diamond turns into carbon dioxide. That is why, unlike coal, after burning a diamond, not even ash remains. Experiments by scientists have confirmed another property of diamond: in the absence of oxygen, diamond does not burn, but its molecular structure changes. At a temperature of 2000°C, graphite can be obtained in just 15-30 minutes.
Diamond is a precious stone, but its properties were only appreciated by physicists in the 16th century. And this despite the fact that the stone was found several centuries earlier. Of course, in order to assess the full significance of the mineral, it was necessary to conduct many experiments. They provided information about the hardness of the stone, the melting point of the diamond, and other physical characteristics. But since then, the stone has been used not only as a beautiful accessory, but also for industrial purposes.
The assessment was carried out in special laboratories. And as a result, the chemical composition of diamond, the structure of its crystal lattice was clarified, and several phenomena were discovered.
Melting Diamond
History[edit | edit code]
| Pre-classic version of Java Edition | ||
| rd-160052 | The stone has been added to the game. | |
| Classic version Java Edition | ||
| 0.26 SURVIVAL TEST 3 | Creeper explosions no longer destroy rock or rock-like blocks. | |
| 0.27 SURVIVAL TEST 10 | When you mine a stone, a cobblestone drops out. | |
| Indev version of Java Edition | ||
| A pickaxe is required to mine stone. | ||
| Cobblestone can now be smelted into stone. | ||
| Java Edition Beta | ||
| 1.8 | You can craft stone bricks from stone. | |
| Official release of Java Edition | ||
| 1.0.0 | Beta 1.9 Prerelease | The stone will be formed if lava flows from above onto the water. In previous versions, lava replaced water and continued to fall. |
| Beta 1.9 Prerelease 5 | The texture has been slightly changed from to . | |
| 1.4.2 | 12w38a | Added new sounds for placing, mining, and walking on stone. |
| 1.5 | 13w01a | The stone is used when crafting loading funnels and red comparators. |
| 13w02a | The stone is no longer used when crafting loading hoppers. | |
| 1.8 | 14w02a | Three new naturally generated stone variants have been added: granite, andesite and diorite, as well as polished versions of all three. |
| 14w27b | Stone textures are rotated randomly, making large areas of stone less monotonous. | |
| 1.14 | 18w44a | Changed stone texture. |
| 0.1.0 | The stone has been added to the game. | |
| 0.9.0 | build 1 | Added granite, diorite, andesite, as well as their smooth versions. |
| Official release of Bedrock Edition | ||
| 1.9 | beta 1.9.0.0 | Added polished stone. |
| Legacy Console Edition | ||
| The stone has been added to the game. |
History[edit | edit code]
| Official release of Java Edition | ||
| 1.14 | 18w44a | Added smelting furnaces. Only available in the Creative Inventory. The melting furnace interface is only available in Observation mode. |
| 18w48a | Melting furnaces appear in villages located on the plains. | |
| 18w50a | Melting Furnaces are now fully functional and can be crafted. | |
| 19w03a | Added sounds for the smelting furnace. | |
| 19w11a | Now residents use it as a workplace. | |
| Official release of Bedrock Edition | ||
| 1.9 | beta 1.9.0.2 | Added smelting furnaces to experimental gameplay. |
| 1.10 | beta 1.10.0.3 | Added a recipe for crafting a smelting furnace. |
| Melting furnaces are now generated in new houses of village armorers. | ||
| 1.11 | beta 1.11.0.1 | Melting furnaces are now fully functional. |
| PlayStation 4 Edition | ||
| The Melting Furnace has been added to the game. |
[edit] Links
| Sacred magical knowledge from the atsral annals. | |
| Numerology | • 25th frame • • • 2012 |
| Exercises | Astrology • Voodoo • Homeopathy • Dolboslavie • Magic • New Age • Raciology • Satanism • Socionics (Sociotypes) • Telegony • Thelema • Theory of Intelligent Fall • Chaosism • Emergence |
| Sorcerers | Ache666 • Peacedoorball • Sherak • Akinator • Akhinevich • Bellikum • Valarat • Vanga • Varrax • Gariaev • Globa • Grabovoi • Dobrolyubov • Kazhdan • Kashpirovsky • Kochergin • Lavey • Levashov • Lotov • Maslov • Messing • Moptyuk • Muldashev • Navosvet • Nostradamus • Oleg T. • Ra-Hari • Ragnvald • Rasputin • Gray Lake • von Sievers • Skavr • Sklyarov • Slyusarchuk • Takayanebo • Tesla • Trekhlebov • Healers • Chashikhin • Chudinov • Chumak • Yarovrat |
| Witches (Witch Hunt) | Deathwisher • Leyla 22 • Aryan • Lambdadelta • Nahema • Nibaal |
| Creatures | Anhuman • Astralopithecus • Vampire • Dwarf • Jumping Jack • Dragon • Zombie • Alien • Kyshtym Dwarf • Popobava • Ghost • Reptilian • Swiborg • Sasquatch • Succubus • Thin Man • Tulpa • Man-sheep • Chupacabra |
| Inquisitors | Criminal gang "Science freaks" • Randy |
| Items and places | Artifacts • Atlantis • Atsral • Bermuda Triangle • Mount Kailash • Horoscope • Area 51 • Dyatlov Pass • The Rod • The Amityville Horror • Yuggoth |
| Orders and sects | Ahnenerbe • God's Will • Unification Movement • Mages • Freemasons • Rejuvenating Apple • Monolith • Order of the Guardians of Death • Scientology • Secret • Black Comintern |
| Battle magic | Battle of Psychics • Charmed • Duel of the Century • Spinor • Fireball |
| Literature | Bulletin of Healthy Lifestyle • Castaneda • Lovecraft • Petukhov • Guide to the Corridors of Hell • Voynich Manuscript |
| Concepts and expressions | Backmasking • It's Magic • Libastral • Sound drugs • Immortality • Magic rabbit • Horoscope • Involtation to egregor • Low-contrast inscriptions • Invisibility • Obsession • Passionarity • Pentagram • Pyramid (Pyramidosrach) • Systematicity • Teleportation • Philosopher's Stone • Chthonic • Miracle • Bnima • Electronic voice phenomenon • This is a very powerful witchcraft • The Mandela effect |
| Near-scientific | ASMR • Hypnosis • Information field of the Universe • Stone casting • Clinical death • Lucid dreams • Sleep paralysis • Torsion fields • Rolling ball phenomenon • Holotropic breathing |
[edit] Betonoids
| The columns were made in sections and were installed in the same way, then polished - this is a casting (possibly also sectional) - a rough column covered with granite plaster. They poured it sectionally. |
The resulting “granite” during the experiment
And I wanted it to turn out like this
Betonoids
(aka
litiki
,
nepromesiki
) - a dead-end branch of armchair experts who devoutly believe in some ancient secret nano-miracle technologies of granite and other stone casting, thanks to which the past civilization cast all the ancient buildings in the world - from the Egyptian pyramids to the embankments of St. Petersburg. A separate line is the opinion that the structures were cast not in ancient times, but in the recent past by some secret service for falsifying the history of a planetary scale. According to the law of the genre, these historical stories are kept secret from the cattle for many years, and only today a small percentage of “intellectuals” realize the real state of affairs, despite the total conspiracy, conspiracies and mutual responsibility of the dictatorship of the world government/ZOG.
What is typical is that most political scientists are familiar with the process of pouring concrete only speculatively, at best, peeping from behind a construction fence or watching a couple of videos - armchair work is clearly visible in their amateurish statements and ideas about the properties of concrete, which are far from reality.
The discovery that changed the world
Everything that we now consider familiar depended on whether the diamond caught fire or not. Firstly, thanks to Lavoisier's experiment, the phlogiston theory was rejected. According to it, a reaction always requires two substances. One is capable of giving, the other is capable of receiving. It was replaced by the law of conservation of energy: nothing comes from nowhere and nothing disappears into nowhere.
Thanks to this law, it was possible to find out that when burned, a diamond turns into carbon. And this gave us, secondly: if carbon can be obtained from diamond, then the reverse reaction must also exist.
While developing this theory, scientists found that diamond can be synthesized. The discovery had a wide resonance, because the mineral is used in many areas of life. The ability to obtain it artificially is an unlimited supply of an invaluable resource.
Notes
- TSB
- Phys. Rev. Lett. 70, 3764 (1993): Thermal conductivity of isotopically modified single crystal diamond
- Dronova Nona Dmitrievna. Changing the color of diamonds during their processing into brilliants (systematic approach and experimental studies) abstract of a dissertation for the scientific degree of candidate of geological and mineralogical sciences. Specialty 04.00.20 - mineralogy, crystallography. Moscow, 1991
- Yuri Shelementyev, Petr Pisarev
World of Diamonds (Russian). Gemological Center of Moscow State University. — Black diamond is called carbonado. Archived from the original on August 23, 2011. Retrieved September 8, 2010. - Science and Technology, October 14, 2002
- Magazine room | Neva, 2003 N9 | Evgeny Treyvus - Calvary of geologist Popugaeva
- the 1957 Lenin Prize was awarded to other geologists. Only in 1970, Popugayeva was awarded an honorary diploma and the “Discoverer of the Deposit” badge.
- Scientists have declassified an impact diamond deposit in Siberia, Lenta.ru
(September 16, 2012). Retrieved September 18, 2012. - “A large diamond comes from small ones”
- B. F. Danilov “DIAMONDS AND PEOPLE”
- life strategy of a creative person
- Magazine "Universities"
- Technology for the production and purification of detonation diamonds // Solid State Physics, 2004, volume 46, issue 4. — P. 586
- lenta.ru: “New technology will allow you to create diamonds of any size” based on materials from “New Scientist”
- New n-Type Diamond Semiconductor Synthesized
- Ekimov, E. A.; VA Sidorov, ED Bauer, NN Mel'nik, NJ Curro, JD Thompson, SM Stishov (2004). "Superconductivity in diamond". Nature 428
(6982):542-545. DOI:10.1038/nature02449. ISSN 0028-0836. Retrieved 2010-02-22. - Superconductivity in Polycrystalline Diamond Thin Films