Everyone knows such substances as graphite and diamond. Graphite is found everywhere. For example, it is used to make cores for simple pencils. Graphite is a substance that is quite accessible and cheap. But a substance like diamond is extremely different from graphite. Diamond is the most expensive stone, very rare and transparent, unlike graphite. It's hard to believe, but the chemical formula of graphite is the same as that of diamond. In this article we will look at how this is possible.
The concept of allotropy
The concept of “Allotropy” has ancient Greek roots: αλλος - other, τροπος - property. Allotropy is the existence of two or more simple substances of the same chemical element. The concept of allotropy was introduced into science by J. Berzelius in 1841 to designate different forms of existence of elements.
The phenomenon of allotropy implies the possibility of creating a certain amount of different substances from the same element. For example, oxygen and ozone contain only oxygen in their composition. The question of how this is even possible has interested many people for a long period of time. Today, scientists can easily explain all the features of this process.
Not all elements are capable of forming several different simple substances. This ability directly depends on the structure of the molecules. Most often, this phenomenon is observed in elements that have variable oxidation states.
This applies to groups such as:
- non-metals;
- semimetals;
- noble gases;
- halogens.
The causes of allotropy can be of several types. The most likely of them, scientists include the following factors:
- Different numbers of atoms required to form one molecule.
- A different order of conjugation of atoms into one molecule.
- Parallels between electron spins.
- A type of crystal lattice.
In order to clearly understand how the phenomenon of allotropy can exist, it is necessary to consider several of the most remarkable examples that are widely found in nature.
Examples of allotropy:
When metals are heated during the transformation process, heat is absorbed, and the change in lattice structure occurs at the same temperature. Many metals are subject to allotropic modifications, for example, titanium, iron, tin, etc. When heated to +1390 ºC, iron is characterized by a face-centered lattice. An increase in temperature to +1540 ºС leads to a restructuring to a centered cubic structure.
What are allotropic substances?
Allotropic substances are a very important concept in chemistry. This is the basis of the basics, which allows you to distinguish substances from each other.
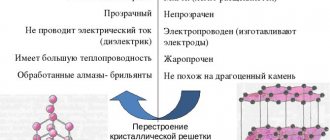
At school, allotropic substances are studied using the example of graphite and diamond, as well as their differences. So, having studied the differences between diamond and graphite, we can conclude that allotropy is the existence in nature of two or more substances that differ in their structure and properties, but have a similar chemical formula or belong to the same chemical element.
Allotropic modifications of carbon
Allotropic modifications of carbon differ most radically from each other in their properties, from soft to hard, etc.
Discovery history:
Carbon in the form of coal, soot and soot has been known to man since time immemorial; about 100 thousand years ago, when our ancestors mastered fire. Probably, very early people became acquainted with allotropic changes in carbon - diamond and graphite, as well as fossil coal. It is not surprising that the combustion of carbon-containing substances was one of the first chemical processes to interest man.
The element was fire, the phenomenon accompanying combustion; In ancient teachings about the elements, fire usually appears as one of the elements. At the turn of the XVII-XVIII centuries. The phlogiston theory arose, put forward by Becher and Stahl. This theory recognized the presence in each combustible body of a special elementary substance - a weightless fluid-phlogiston, which evaporates during the combustion process.
Since when a large amount of coal is burned, only a little ash remains, phlogistics believed that coal was almost pure phlogiston. This is what explained, in particular, the “phlogisticating” effect of coal—its ability to restore metals from “limes” and ores. Later phlogistics, Reaumur, Bergman and others, already began to understand that coal is an elementary substance. However, “clean coal” was first recognized as such by Lavoisier, who studied the process of combustion of coal and other substances in air and oxygen.
In the book “Method of Chemical Nomenclature” by Guiton de Morveau, Lavoisier and Fourcroix (1787), the name “carbon” appeared instead of the French “pure coal”. Under the same name, carbon appears in the “Table of Simple Bodies” in Lavoisier’s “Elementary Textbook of Chemistry.” In 1791, the English chemist Tennant was the first to obtain free carbon; he passed phosphorus vapor over calcined chalk, resulting in the formation of calcium phosphate and carbon. It has been known for a long time that diamond burns without leaving a residue when heated strongly.
Back in 1751, the French king Francis I agreed to provide diamond and ruby for burning experiments. It turned out that only diamond burns, and ruby (aluminum oxide with an admixture of chromium) can withstand prolonged heating at the focus of the ignition lens without damage. Lavoisier carried out a new experiment on burning diamonds using a large incendiary machine and came to the conclusion that diamond is crystalline carbon.
The second allotrope of carbon - graphite in the alchemical period was considered a modified lead luster and was called plumbago; It was only in 1740 that Pott discovered the absence of any lead impurity in graphite. Scheele studied graphite (1779) and, being a phlogistician, considered it a special kind of sulfur body, a special mineral coal containing bound “aerial acid” (CO2) and a large amount of phlogiston. Twenty years later, Guiton de Morveau turned diamond into graphite and then into carbonic acid by careful heating.
The international name Carboneum comes from the Latin carbo (coal). This word is of very ancient origin. It is compared with cremare - to burn; root car, cal, Russian gar, gal and gol, Sanskrit cra means to boil, cook. The word “carbo” is also associated with the names of carbon in other European languages (carbon, charbone, etc.). The German Kohlenstoff comes from Kohle - coal.
Old Russian ugorati, or ugarati (to burn, scorch) has the root gar, or mountains, with a possible transition to gol; coal in Old Russian yugal, or coal, of the same origin. The word diamond comes from the ancient Greek - indestructible, unyielding, hard, and graphite from the Greek - I write. At the beginning of the 19th century. The old word coal in Russian chemical literature was sometimes replaced by the word “carbonate” (Shere, 1807; Severgin, 1815); Since 1824, Soloviev introduced the name carbon.
Structure of carbon atoms
There are 6 electrons in a carbon atom, of which 4 electrons are located in the outer electron layer (see Fig. 32):
The carbon atom lacks 4 electrons before completing its outer electron layer. Therefore, in their compounds with metals and hydrogen, carbon atoms exhibit a negative oxidation state of –4, for example, aluminum carbide.
In compounds with more electronegative elements, carbon atoms exhibit positive oxidation states +4 and +2, for example - carbon dioxide and CO - carbon monoxide.
Structure and physical properties of simple substances
Like oxygen, sulfur and phosphorus, carbon forms several allotropic modifications. The most famous of them are graphite and diamond.
Graphite is a dark gray substance consisting of carbon atoms, which are arranged in layers (Fig. 88). These layers are relatively loosely bonded to each other, so the graphite is soft and can be separated into individual flakes. The ability of graphite to leave a line when rubbed is the basis for its widespread use in the production of pencils.
Soot, charcoal, obtained by heating wood without access to air, and coke , obtained from coal, are products with a high carbon content. Charcoal has the ability to absorb (adsorb) vapors, gases and substances from liquid solutions. This is explained by the fact that it has a large number of pores and, therefore, has a large surface area. Place crushed charcoal in a glass with litmus solution (Fig. 89). After some time, the liquid in the glass will become discolored as the charcoal absorbs the litmus.
The sorption properties of charcoal are widely used in gas masks, in the chemical industry, for decolorization and purification of sugar syrup, oil, fats, wines, drinking water, and also in medicine.
Unlike graphite in diamond (Fig. 90), each carbon atom is connected to other atoms by four chemical bonds directed towards the vertices of the tetrahedron. All bonds between carbon atoms are identical, short in length and very strong. Therefore, diamond is the hardest natural substance. Diamond forms transparent crystals that highly refract light. Cut diamonds are called brilliants.
Graphite conducts electricity well, while diamond is an insulator.
Other allotropic modifications of carbon are also known: carbyne, fullerenes, graphene. You will become acquainted with them in the 11th grade chemistry course.
The fact that different allotropic modifications of carbon consist of atoms of the same element can be verified by burning them in oxygen. When burned, they all form the same product - carbon monoxide (IV) and nothing else. In addition, equal masses of graphite, diamond, carbyne and fullerene will give the same amount of carbon dioxide.
At the end of the 18th century. the famous French chemist Lavoisier, together with his colleagues, bought a small diamond and burned it in a huge “incendiary machine” (see figure) using focused solar rays. In this case, only one product was formed - carbon dioxide CO2. Lavoisier obtained the same gas by burning charcoal. These experiments allowed the scientist to conclude that diamond and coal have “the same beginning.”
Under certain conditions, it is possible to transform one modification of carbon into another. Thus, when heated strongly without access to air, the diamond turns black and turns into graphite. Graphite at temperatures above 2000 °C and a pressure of about 100,000 atm turns into diamond. This process is used to produce artificial diamonds that have technical applications.
When writing equations for chemical reactions, various modifications of carbon are designated by the letter C.
Chemical properties of carbon
Carbon reacts with other substances, usually when heated.
The oxidizing properties of carbon manifest themselves when it interacts with metals at high temperatures:
The resulting compounds are called carbides.
Carbon exhibits reducing properties when interacting with oxygen, forming carbon monoxide (II) when there is a lack of oxygen:
or in its excess - carbon monoxide (IV):
The reducing properties of carbon also appear in reactions with complex substances. Thus, by reacting carbon with iron(III) oxide, metallic iron is obtained:
This is one of the very first chemical processes mastered by man.
- Of the allotropic modifications of carbon, the best known are graphite and diamond.
- When interacting with other substances, carbon can exhibit both reducing and oxidizing properties.
Carbon oxides
Among the inorganic compounds of carbon, the most important are its oxygen compounds: oxides, carbonic acid and its salts.
Carbon(II) monoxide
The model of the carbon monoxide molecule is presented in Figure 91. It belongs to non-salt-forming oxides, since it does not interact under normal conditions with either acids or alkalis.
Carbon monoxide (II) CO is formed during incomplete combustion of fuel (wood, peat, coal) and can enter the air. When a person inhales such air, poisoning occurs (fumes), which is why CO is called carbon monoxide. Carbon monoxide is also found in tobacco smoke and car exhaust. Carbon monoxide (II) is a strong poison! When inhaled, it binds to hemoglobin in the blood more strongly than oxygen, thereby blocking the transport of oxygen in the body. Oxygen starvation occurs, accompanied by headache and loss of consciousness. Severe poisoning can result in death. A person affected by carbon monoxide should be taken out into fresh air as quickly as possible and given medical assistance.
Carbon monoxide (II) burns in air with a bluish flame, releasing a large amount of heat, turning into carbon dioxide:
In this reaction, carbon(II) monoxide exhibits reducing properties.
The reducing properties of carbon(II) monoxide are also manifested in its reactions with metal oxides. The products of these reactions are metal and carbon dioxide:
This reaction is used industrially to obtain metals from ores.
Carbon monoxide (II) serves as a starting material for the production of large amounts of organic substances. At the same time, it is one of the most dangerous air pollutants.
Carbon monoxide
You are already familiar with carbon monoxide (IV), or carbon dioxide. The molecular model and graphical formula of this oxide are shown in Figure 92.
The molar mass is approximately 1.5 times the average molar mass of air (29 g/mol), so carbon dioxide is heavier than air. Carbon dioxide does not support breathing, so in its atmosphere animals and humans die from lack of oxygen.
When cooled or under increased pressure, carbon dioxide solidifies, forming a white crystalline substance resembling snow (“dry ice”). In this form, it is widely used as a cooling agent for storing perishable products such as ice cream.
Carbon dioxide can be produced by reacting carbon with oxygen when heated:
Carbon monoxide (IV) is formed by the combustion of various organic substances (methane, alcohol, coal, etc.). The reaction is accompanied by the release of a large amount of heat, so the combustion of these substances is used to produce thermal energy. Carbon dioxide is also formed during the respiration of living organisms and during decay.
In industry, carbon oxide (IV) is produced by burning limestone
In the laboratory it is possible to obtain by the action of acids on carbonates, for example calcium carbonate (Fig. 93):
Carbon dioxide is an acidic oxide; it dissolves slightly in water, forming weak carbonic acid:
This is what causes the sour taste of carbonated and some mineral waters.
With basic oxides and alkalis it forms salts of carbonic acid - carbonates:
When passing calcium hydroxide (limewater) through a solution, the solution becomes cloudy and a precipitate of calcium carbonate precipitates (Fig. 94):
This reaction is qualitative to carbon dioxide.
Carbon(II) monoxide is characterized by reducing properties.
Carbon(IV) monoxide - Carbon dioxide is an acidic oxide. When dissolved in water it forms weak carbonic acid.
Carbon dioxide reacts with basic oxides and bases.
Carbonic acid and its salts
In a carbonic acid , the carbon atom is connected to three oxygen atoms by one double bond and two single C-OH bonds. The molecule model and graphical formula of carbonic acid are presented in Figure 95.
In aqueous solutions, carbonic acid is a very fragile substance. When you try to isolate it from solution, it almost completely decomposes into carbon dioxide and water:
At the same time, the solution in water tastes slightly sour, and when litmus is added to the solution, it turns pink. Therefore, a solution of carbon monoxide (IV) in water can be considered a solution of carbonic acid.
In 2011, researchers from the Technical University of Vienna and the University of Innsbruck (Austria) obtained carbonic acid in the form of a white solid that is stable in air at temperatures below –30 °C.
Carbonic acid is a weak dibasic acid and dissociates stepwise in an aqueous solution. At the first stage of dissociation, a hydrogen ion and a bicarbonate ion are formed
The prefix hydro- in the name of an acidic residue indicates the presence of a hydrogen atom in its composition. Salts containing such an acidic residue belong to the so-called acid salts and are called bicarbonates .
In the second step, the bicarbonate ion dissociates to form a hydrogen ion and a carbonate ion
Salts containing the carbonate ion are medium and are called carbonates .
Chemical properties of carbonic acid salts
Salts of carbonic acid, except for the carbonates of most alkali metals, decompose when heated to release carbon dioxide:
Carbonates and bicarbonates, as salts of a very weak acid, react with all stronger acids to release carbon dioxide. If you drop a solution of hydrochloric acid onto a piece of chalk, which is calcium carbonate, you will see a characteristic effervescence due to the rapid release of carbon dioxide (Fig. 96):
This test can be carried out with both solid carbonates and their solutions. The above reaction is considered as a qualitative reaction for the determination of carbonate ions.
For soluble carbonates, the equation for the qualitative reaction to ions can be written in abbreviated ionic form:
Carbonates can be used to neutralize acids, since when they interact with acids, hydrogen ions bind. For example, ground limestone, consisting mainly of CaCO3, and dolomite flour (CaCO3 MgCO3) are added to soils when they are too acidic. Wood ash plays a similar role due to the potassium carbonate it contains.
Transformations of carbonates and bicarbonates
If you pass carbon dioxide through a solution of calcium hydroxide (see Fig. 94), then clouding of the solution will be observed due to precipitation:
With further passage of carbon dioxide, the calcium carbonate solids will dissolve and the liquid will become clear again. Water-soluble is formed :
When heated, calcium bicarbonate turns into carbonate:
In nature, the occurrence of processes involving carbon dioxide, water and limestone, chalk, marble (all these substances are CaCO3 in chemical composition) leads to their gradual dissolution due to transformation into bicarbonate. As a result, huge cavities and caves appear in the earth's crust. Calcium bicarbonate transforms into calcium carbonate, forming stalactites and stalagmites (see figure).
Application of carbonic acid salts
One of the most widely used carbonic acid salts is sodium carbonate. It is known as soda ash and crystalline soda.
Soda ash is used in the production of soap, glass, for the production of inorganic dyes, in the production of aluminum, etc.
Acid salt - sodium bicarbonate is called baking soda. Baking soda is used in everyday life and in the food industry. If you add baking soda to the dough, then when baking products it decomposes, releasing carbon dioxide. This leads to loosening of the dough, and products made from it become more fluffy and porous.
Calcium carbonate, which exists in nature in the form of marble and limestone, is widely used in construction as facing and architectural building materials. In Figure 97 you see the Minsk metro station “Grushevka”, during the construction of which marble finishing was used.
Weak carbonic acid H2CO3 is formed when carbon dioxide is dissolved in water.
Carbonic acid forms two series of salts: acidic - hydrocarbonates and medium - carbonates.
Carbonates and hydrocarbonates are capable of mutual transformations.
Carbonates, as salts of a weak acid, react with all stronger acids to release carbon dioxide.
Salts of carbonic acid, except for the carbonates of most alkali metals, decompose when heated, releasing carbon dioxide.
The concept of organic substances
The total number of substances known today is enormous - there are more than 150 million of them! The vast majority of them are organic substances. They received this name because many of them were isolated from organisms .
One of the first such substances was probably fats. Early man, a hunter-gatherer, learned about them through the process of cooking. By roasting hunted animals over a fire or grinding the seeds of certain plants, he observed the release of viscous liquids that had similar properties. These substances were very nutritious and gave the body a lot of strength. People have long learned to extract fats from natural objects and have been using them as food or materials for obtaining other useful substances for many centuries. Today, everyone is familiar with fats of animal origin - pork fat, butter, as well as fats extracted from plants - sunflower, olive, flaxseed, palm, peanut and other oils.
While cooking meat over a fire, an ancient man accidentally made an important discovery. It turned out that drops of fat, falling on wet ash and cooling, gradually turned into a dense mass that foamed in the water and washed the dirt off the hands well. This is probably how people first became acquainted with soap, without which it is impossible to imagine our lives. Of course, today soap is made in a different way, but its basis is still fats.
Another important observation was made in ancient times. When crushed stems of one type of cane were squeezed, a liquid with a pleasant sweet taste was released. When this liquid was evaporated, a solid, even sweeter substance was obtained, called sugar. And from the liquid squeezed from potato tubers, a white substance called starch was isolated. Subsequently, it was found that sugar and starch are representatives of a large class of substances - carbohydrates.
By grinding grains of various cereals, people obtained flour, which, when mixed with water, forms dough for baking bread. In the first half of the 18th century. Gluten, an elastic and elastic mass, was isolated from dough for the first time. Subsequently, it turned out that it is a mixture of special substances - proteins, which can be of plant (gluten) and animal (chicken egg white) origin.
The vast majority of organic compounds currently known are non-natural substances - they are produced artificially in chemical laboratories or chemical plants (Fig. 98, 99). They are part of various valuable materials - synthetic fibers and rubbers, plastics and medicines, detergents and dyes, pesticides and fertilizers, explosives. Every week, thanks to scientific research, the number of organic substances increases by approximately 10,000.
All organic substances have a number of common properties that are unlike the properties of inorganic substances. How do organic substances differ from inorganic substances?
Firstly, in an amount that is more than 149 times greater than the number of inorganic compounds. Organic substances are incredibly diverse, and the number of classes of these compounds is tens of times greater than that of inorganic substances. The large number of organic substances and the diversity of their classes are due to the peculiarities of their composition and structure, which you will become familiar with in the next paragraph.
Secondly, the molecules of all organic substances necessarily include carbon atoms associated with atoms of a small number of elements - most often hydrogen, oxygen, nitrogen, sulfur, halogens, phosphorus. In this way, organic substances differ sharply from inorganic substances, which may contain atoms of all known chemical elements. Note that such simple carbon compounds as its oxides CO and carbonic acid and its salts are traditionally classified as inorganic substances.
Thirdly, many organic compounds are thermally unstable and, even at relatively low temperatures, decompose with the formation of carbon, that is, they become charred. When burned in oxygen, they form carbon dioxide and water. As for inorganic substances, most of them are thermally stable or decompose at very high temperatures. The products of their combustion in oxygen are a wide variety of substances.
Fourthly, organic substances are characterized by covalent polar and covalent nonpolar bonds. This also distinguishes organic substances from inorganic substances, which, in addition to the indicated types of bonds, also have ionic and metallic bonds.
Fifthly, almost all organic substances are compounds of molecular structure with low melting points. They are characterized by molecular crystal lattices. At the same time, most inorganic substances are compounds of non-molecular structure with high melting points. They are more characterized by atomic or ionic crystal lattices.
Despite the significant differences between organic and inorganic substances, their division into two groups is conditional. Both substances are formed and transformed in accordance with the same laws of nature. Organic and inorganic substances are united by their ability to transform each other. For example, as a result of photosynthesis, the organic substance glucose is formed from inorganic substances - carbon dioxide and water. Being a component of food, in human and animal organisms it again turns into its original inorganic compounds. This interconversion is the basis of the carbon cycle in nature.
Carbon is the basis of organic compounds
You have more than once observed clouds of thick black smoke escaping from the exhaust pipe of a faulty car running on diesel fuel (Fig. 100). It slowly rises up and mixes with the air, polluting it. Where does this smoke come from? Why is it black, although the liquid diesel fuel that the car is fueled with is transparent and almost
colorless? Here's the thing. The composition of this fuel includes various compounds of carbon with hydrogen, the so-called hydrocarbons, for example. When a working engine is running, they are mixed with air and completely burn, releasing heat, forming carbon dioxide and water, for example:
If the engine is faulty, then some of the hydrocarbons do not burn completely: oxygen from the air binds only with H atoms, and the remaining carbon atoms form a simple substance carbon, for example:
Carbon in the form of tiny black particles is forcefully ejected from the engine exhaust gases to the outside, forming a cloud of black smoke.
The formation of carbon from organic substances can also be observed in the school laboratory. Let's conduct an experiment. Pour some white glucose powder into a test tube and heat it in the flame of an alcohol lamp. First, the glucose will melt and turn into a viscous liquid, which, with further heating, will begin to foam and darken. After some time, droplets of water will form on the walls of the test tube, and a black solid substance - carbon - will remain at the bottom (Fig. 101).
Carbon is also formed by strong heating of other organic substances and materials based on them. What do the emission of black smoke from car exhaust pipes and the blackening of glucose when heated indicate? Of course, about the fact that the composition of molecules of organic substances
includes carbon atoms. Proof of this is the fact that with the complete combustion of organic substances in oxygen, carbon dioxide is always formed along with other substances
Carbon atoms bonded to atoms of other elements are present in the molecules of all organic substances without exception. For this reason, the branch of chemistry that studies these substances—organic chemistry—is called the chemistry of carbon compounds.
Why, out of 118 chemical elements, is carbon the basis of all organic substances? The answer to this question lies in the structural features of the atom of a given element.
Since carbon is a chemical element with atomic number 6, located in the second period of the periodic table, its atom has 6 electrons distributed over two electron layers. Since carbon is a group IVA element, the outer electron layer of its atom contains 4 electrons:
The electronic structure of an atom determines its following features.
1. Due to the presence of 4 electrons on the outer electron layer of the carbon atom, it does not have a pronounced ability to give or accept electrons and thus turn into ions. Therefore, carbon atoms form not ionic, but only covalent bonds, characteristic of molecules of organic substances.
2. Due to the fact that the radius of the carbon atom is small, the covalent bonds it forms are very strong. Carbon atoms form such covalent bonds with the atoms of most known chemical elements.
3. Since there are 4 electrons on the outer electron layer of the carbon atom, it exhibits a valency equal to IV - it forms four covalent bonds with other atoms. These can be four single bonds; two single and one double; two doubles; one single and one triple bond:
Here are examples of molecules of organic substances with such bonds:
4. Carbon atoms, unlike atoms of other elements, are capable of joining into chains of any length. They can be unbranched, branched and closed in cycles (Fig. 102):
And these are examples of molecules of organic substances with such chains of carbon atoms (Fig. 103):
Thus, atoms of only one chemical element - carbon - can combine both with atoms of other elements and with each other, forming chains or cycles in which carbon atoms are connected to other atoms by single, double or triple bonds. This uniqueness of carbon atoms is the basis for an incredibly large number of organic compounds and the diversity of their classes.
- Carbon atoms form covalent bonds, characteristic of molecules of organic substances.
- Carbon atoms form chemical bonds with atoms of most known elements.
- Carbon atoms are connected to other atoms by four covalent bonds (single or multiple).
- Carbon atoms are capable of connecting with each other into chains of any length, which can be unbranched, branched or closed in cycles.
The importance of organic substances in nature and human life
The world of organic substances is huge and diverse. Currently, according to their origin, they are divided into two groups. The first group includes organic compounds of natural origin that are part of all living organisms - humans, animals, plants, etc. They are also found in inanimate nature in the form of oil and natural gas. The second group consists of organic substances of non-natural (artificial) origin. Let's get acquainted with the role of organic substances in nature and human life.
Organic substances of natural origin
Organic substances of natural origin participate in all processes occurring in living organisms. The most important of them are proteins, fats, carbohydrates, vitamins, various acids, enzymes and hormones.
Proteins are vital substances whose molecules are chains of many thousands of atoms of carbon, hydrogen, oxygen, nitrogen and sulfur. In addition to the chicken egg protein that we have known since childhood, several million other proteins are known. They are found in the bodies of all living organisms and perform many functions. For example, proteins are part of muscles, bones, blood, form cartilage, skin, hair, nails, horns, hooves, feathers, scales (Fig. 104). Proteins are involved in muscle contraction processes and protect the body from infections. In living organisms, some proteins play the role of enzymes and hormones that regulate all vital processes.
In plant organisms, proteins are found in greatest quantities in seeds, where they are stored. The seeds of peas, beans, soybeans, and wheat grains are especially rich in protein.
Proteins are an important source of energy for humans and animals; they are part of food.
Some natural poisons are protein in nature and have a toxic effect on humans. These are proteins from the venom of snakes, some spiders, bees, wasps, as well as proteins from poisonous mushrooms, such as toadstools and fly agarics.
Along with proteins, nucleic acids . Their molecules, consisting of a huge number of carbon, hydrogen, oxygen, nitrogen and phosphorus atoms, are “templates” by which organisms synthesize the necessary proteins. Nucleic acids are a kind of memory devices with the help of which each type of living organism passes on the “recording” of the structure of its proteins from generation to generation.
Fats are complex organic substances containing carbon, hydrogen and oxygen atoms. They are found in human bodies, animals, plants, etc. Everyone knows fats of animal origin, for example pork, beef, lamb fat, butter (Fig. 105). Vegetable fats are called oils. These include sunflower, flaxseed, rapeseed, olive, peanut, palm and other oils. They accumulate in seeds or fruits of plants. You can check this by placing a sunflower seed on a piece of paper and pressing it firmly. An oily stain will appear on the paper.
Fats are the most important source of energy for humans and an integral part of food. By forming fat capsules, fats protect internal organs from shock and protect the body from hypothermia. The fats secreted by the sebaceous glands of the skin make human skin soft and elastic, and hair shiny. Together with proteins, fats are a reserve building material from which new body cells are formed.
Carbohydrates are complex organic substances whose molecules consist of carbon, hydrogen and oxygen atoms. Carbohydrates are formed in green plants during photosynthesis from carbon dioxide and water. They are part of the cells and tissues of all plant and animal organisms and, by mass, make up the bulk of organic matter on Earth.
Green plants annually absorb approximately 200 billion tons of carbon dioxide CO3 from the atmosphere through the process of photosynthesis. At the same time, about 130 billion tons of oxygen O2 enters the atmosphere and 50 billion tons of carbohydrates are synthesized.
Animal organisms are not able to synthesize carbohydrates, so they obtain them from plant sources. Carbohydrates include, for example, glucose, fructose, sucrose, starch, cellulose, etc. Glucose, fructose and sucrose are found in the juice of vegetables and fruits, giving them a sweet taste. Glucose is an essential component of the human body. Sugar beets and sugar cane are rich in sucrose - the main sources of sugar. Starch accumulates in tubers, fruits, and plant seeds. Thus, potato tubers contain up to 24% starch, wheat grains - up to 64%, rice - 75%, corn - 70%. Glucose, fructose, sucrose and starch are important sources of energy for humans. They are easily digestible and are included in food products. Cellulose (fiber) is a carbohydrate that makes up the cell walls of all higher plants. Cellulose is familiar to every person and is found literally at every step. Poplar fluff and dandelion parachutes, cotton wool made from cotton seeds (Fig. 106), flax, straw, paper - all this is almost pure cellulose. It is part of such an important material as wood. In mammals, which includes humans, cellulose is not digestible. However, it is the main food of many herbivores, such as cows, sheep, horses, and deer.
Vitamins are organic substances that do not supply energy to the body, but are needed in small quantities to maintain life. Natural sources of vitamins are vitamin-rich plants (rose hips, citrus fruits, parsley, onions, cabbage, carrots, currants, rowan, sea buckthorn, etc.), as well as some food products of animal origin. Many vitamins today are obtained synthetically.
Vitamins enter the body with food and are involved in almost all processes occurring in our body. They are necessary for the normal functioning of the endocrine glands, increasing mental and physical performance, and the body’s resistance to the effects of adverse environmental factors (heat, cold, infections, poisoning). Currently, about 20 different vitamins are known. This is, for example, vitamin C - the familiar “ascorbic acid”, as well as vitamins, etc. A lack of vitamins, as well as their excess in the body, cause various diseases.
Diamond
Carbon has many allotropic modifications. The main ones are graphite, diamond, carbine .
They differ from each other in physical, chemical properties, and crystal lattice structure. The hardest modification, diamond, is used for the manufacture of industrial tools. According to modern theoretical concepts, nothing in the world can be harder than diamond - such is its crystal lattice. We can say that diamond is the hardest mineral in the world. Diamond melts at high temperatures, from 3700 to 4 thousand degrees. But even earlier, at 850 degrees, it begins to burn, and without air access, when it reaches half the melting temperature, it turns into graphite.
Mining history
Diamonds were not always precious stones that had value and a scale of value. In nature, this pebble is unattractive - a simple rough piece of glass. It is the cut that gives it its value. Everything changed in the 19th century, when diamond seekers settled on the farm of the de Beers brothers in South Africa near the modern city of Kimberley. There were a lot of stones on these lands. Their real industrial production became associated with the name of Cecil Rhodes.
Cecil Rhodes became a monopolist in the diamond market, which was facilitated by the Rothschilds, and diamonds became a consumer product available not only to kings. De Beers' monopoly position was shaken only in the middle of the 20th century thanks to antitrust laws in the USA and the beginning of mass production in countries where there were no opportunities to capture the market in principle - for example, in the USSR. There are several ways to cut diamonds, in which they best demonstrate their properties of color play. The original shape of the stone also plays a role, since the cutter tries to reduce its losses to a minimum.
The most common diamond shapes are:
- round, 57 edges;
- oval;
- "pear";
- "marquise";
- radiant;
- square;
- "princess".
Diamond Structure:
One carbon atom is surrounded by four more atoms in the form of a tetrahedral triangle or pyramid. Each atom is the same distance from each other. The bonds between atoms are very strong, which is why diamond is so hard and durable. Another property of diamond is that it can conduct light, unlike graphite.
Diamond: history and properties of the mineral
The history of the diamond is very unusual. It is believed that the first diamond was found in India. At that time, humanity was never able to understand the full power of this stone. Geologists only knew that this stone was very hard and durable. Until the 15th century, diamonds were worth much less than emeralds and rubies. And only then an unknown jeweler, while working with the stone, gave it a beautiful cut, which later became known as a diamond cut. It was then that the stone showed itself in all its glory.
Diamonds are mainly used in industry. This mineral is the strongest in the whole world, which is why abrasives, cutters for processing durable metals, and much more are made from it.
As we already know, the chemical formula of graphite is C, and diamond has the same formula.
Graphite
Graphite is a mineral from the class of native elements, one of the allotropic modifications of carbon. The structure is layered. The layers of the crystal lattice can be differently positioned relative to each other, forming a number of polytypes, with symmetry from hexagonal system (dihexagonal-dipyramidal) to trigonal (ditrigonal-scalenohedral).
The layers are slightly wavy, almost flat, and consist of hexagonal layers of carbon atoms.
Mining history:
In the 60s of the 16th century in England. Local shepherds, who found deposits of a strange black-shiny material, at first mistook it for lead, but, realizing that they couldn’t cast bullets from it, they began to break off pieces of black stone and mark their sheep with it. The new material soon attracted the attention of artists and merchants, who quickly established trade in thin plates and pieces of graphite on the English streets. Of course, it was very inconvenient to use - your hands got dirty! I had to wrap the graphite with rope, paper, or even simply press it between boards. This is how the first pencils in a wooden case appeared.
Graphite structure:
Physical properties in graphite vary greatly in directions - perpendicular and parallel to the layers of carbon atoms.
When heated without air access, graphite does not undergo any changes up to 3700°C. At the specified temperature, it sublimes without melting.
Artificial graphite is produced from the best grades of coal at 3000°C in electric furnaces without air access.
Graphite is thermodynamically stable over a wide range of temperatures and pressures, so it is accepted as the standard state of carbon. The density of graphite is 2.265 g/cm3.
There are two known forms of graphite: alpha graphite (has a hexagonal structure and crystal lattice) and beta graphite (has a rhombohedral structure and crystal lattice). In α-graphite, half of the atoms of each layer are located above and below the centers of the hexagon, and in β-graphite, every fourth layer repeats the first.
Alpha graphite can be converted to beta form by mechanical processing. The beta form transforms into the alpha form when graphite is heated above 1300 °C.
Mining and production
The history of mining and trading of these precious stones goes back thousands of years. They were first discovered in India - the exact date is unknown, but the description of the crystal was made back in IV BC. e. Even then it was considered very valuable, even though people of that time did not know how to cut the mineral and only polished it. It is known that diamonds were also popular in ancient Egypt, and they were brought to Europe during the time of Alexander the Great.
They learned to cut crystals in the 17th century, and since then this art has been constantly improved. Over the centuries, new forms and methods of processing the precious mineral appeared, until in 1919 Marcel Tolkowsky invented the ideal cut of diamonds, which is still used today.
In natural conditions
Diamonds are classified as natural minerals. The bulk of the gems - about 90% - are found in kimberlite pipes; some of them can also be found in lamproites and placers. The presence of this valuable mineral in rock is determined using X-rays. At the same time, most of the mined stones are used not for jewelry, but for industry - this is due to the presence in them of a large amount (> 5% overall or > 2% of a substance of one type) of impurities, as well as structural defects. Industrial crystals are of the following types:
- bead - small diamond chips that cannot be cut, used for cutting some refractory materials;
- ballas - fine-grained crystals, the shell of which is harder than the core, usually have a cloudy white or gray color and are used for the production of drill bits;
- carbonado is an opaque or translucent black diamond, similar to coal, usually containing many impurities of iron, graphite or sulfites.
Fullerene
Fullerene , buckyball, or buckyball is a molecular compound that belongs to the class of allotropic forms of carbon and is a convex closed polyhedron composed of an even number of three coordinated carbon atoms.
The unique structure of fullerenes determines their unique physical and chemical properties. In combination with other substances, they make it possible to obtain materials with fundamentally new properties. Mining history:
The discovery of the fullerene resulted from experiments by Smalley and Croteau with an instrument that Smalley had invented to study molecules and atomic clusters. Kroteau was interested in Smalley's proposed laser evaporation technique. With its help, he intended to test his theory about the behavior of carbon in interstellar space. Croteau believed that carbon-rich red giants were capable of emitting complex carbon compounds that could be detected by radio telescopes.
Fullerene structure:
Atom bond Fullerene is a new allotropic form of carbon. Fullerene molecules consist of 60.70 atoms forming a sphere. Crystalline fullerenes are semiconductors. The variety of physicochemical and structural properties of compounds based on fullerenes allows us to talk about the chemistry of fullerenes as a new promising direction in organic chemistry.
Carbon atoms are located at the vertices of regular hexagons and pentagons that make up the surface of a sphere or ellipsoid. The most symmetrical and most fully studied member of the fullerene family is fullerene (C60), in which the carbon atoms form a truncated icosahedron consisting of 20 hexagons and 12 pentagons and resembling a soccer ball.
The next most common is the C70 fullerene, which differs from the C60 fullerene by the insertion of a belt of 10 carbon atoms into the equatorial region of C60, as a result of which the C70 molecule is elongated and resembles a rugby ball in shape. The so-called higher fullerenes, containing a larger number of carbon atoms (up to 400), are formed in much smaller quantities and often have a rather complex isomeric composition. Among the most studied higher fullerenes are Cn, n=74, 76, 78, 80, 82 and 84.
Ionic crystal lattice
As is known, during an ionic chemical bond, one atom gives ions to another and acquires a positive charge, while the receiving atom becomes negatively charged. As a result, oppositely charged ions appear, which make up the structure of the crystal.
An ionic lattice is a crystalline structure, at the nodal points of which there are ions bound by mutual attraction.
Almost all salts have an ionic crystal lattice; table salt NaCl can be considered a typical representative. It is worth remembering if you need to list the physical characteristics of this group. Also, alkalis and oxides of active metals have an ionic lattice.
Properties of substances with ionic structure:
- hardness;
- fragility;
- infusibility;
- non-volatility;
- electrical conductivity;
- ability to dissolve in water.
Examples of substances with an ionic crystal lattice: calcium oxide CaO, magnesium oxide MgO, ammonium chloride NH
4
Cl
, magnesium chloride Mg
Cl
2, lithium oxide
Li
2
O
and others.
Physical properties
Physical properties. Carbon exists in a variety of allotropes with very diverse physical properties. The variety of modifications is due to the ability of carbon to form chemical bonds of different types.
Therefore, different modifications exhibit very different physical properties: a very hard substance, a soft substance that conducts electric current, and many others.
Diamond:
Phys. properties. Diamond and graphite differ sharply in physical properties. Properties. Diamonds are transparent crystals, very hard. Hardness is explained by the structure of its crystal lattice. Graphite is a soft dark gray substance with a Me shine.
Graphite:
Physical properties of graphite. – a soft black substance made of easily exfoliated crystals, – conducts electric current, – high-purity graphite is used in nuclear reactors as a neutron moderator. - the melting point at elevated pressure is 3527° C. - At normal pressure, graphite sublimates at 3780° C.
Fullerene:
Physical properties of fullerene Condensed systems consisting of fullerene molecules are called fullerites. The most studied system of this kind is the C60 crystal, less so is the system of crystalline C70. Studies of crystals of higher fullerenes are hampered by the complexity of their preparation.
Molecular crystal lattice
As in the previous group, this one contains substances with covalent bonds between atoms. But the physical characteristics of these substances are completely different - they easily melt, turn into liquid, and dissolve in water. Why is this happening? The thing is that here crystals are built not from atoms, but from molecules.
A molecular crystal lattice is a structure in whose nodes there are not atoms, but molecules.
Inside molecules, atoms have strong covalent bonds, but the molecules themselves are weakly bonded to each other. Therefore, the crystals of such substances are fragile and easily disintegrate.
The molecular crystal lattice is characteristic of water. At room temperature it is a liquid, but as soon as it is heated to the boiling point (which is relatively low), it immediately begins to turn into steam, that is, it goes into a gaseous state.
Some molecular substances - such as dry ice CO
2, are capable of transforming into gas immediately from the solid state, bypassing the liquid state (this process is called sublimation).
Properties of molecular substances:
- slight hardness;
- low strength;
- fusibility;
- volatility;
- some have an odor.
In addition to water, substances with a molecular crystal lattice include ammonia NH
3, helium He, radon Rn, iodine I, nitrogen
N
2 and others. All noble gases are molecular substances. Most organic compounds (for example, sugar) also belong to this group.
Chemical properties
Diamond:
At ordinary temperatures, carbon is inactive. It can be both a reducing agent and an oxidizing agent. As a reducing agent: Burns in air.
Graphite:
Chemical properties. It forms inclusion compounds with many substances (alkali metals, salts). Reacts at high temperatures with air, burning to carbon dioxide.
Fullerene:
Reduction, nucleophilic addition, cycloaddition, regiochemical multiple addition, halogenation, modification of fullerenes, cluster hydrogenation, addition of radicals, formation of complexes, transition metal oxidation and reactions with electrophilic reagents.
Practical part
Application of allotropic modifications of carbon. Diamond - in industry: it is used to make knives, drills, cutters; in jewelry making. The future is the development of microelectronics on diamond substrates. Graphite – for the manufacture of melting crucibles, electrodes; plastic filler; neutron moderator in nuclear reactors; component of the composition for the manufacture of leads for black graphite pencils (mixed with kaolin) Fullerene - in batteries and electric batteries (fullerene additives); pharmacology (HIV treatment); Solar cells; fire retardant paints.
New allotropic modification:
On August 15, 2022, a group of scientists from IBM and the University of Oxford published a paper presenting data on the successful synthesis of the cyclo[18]carbon molecule. Previously, the existence of cyclocarbons was considered only hypothetical, but now C18 is a representative of a new allotropic modification of carbon.
C18 was obtained by removing carbon monoxide from the C24O6 molecule by the principle of atomic force microscopy on a two-layer sodium chloride surface at a temperature of 5°K (-268.15°C). According to scientists, cyclocarbons may prove useful in computer technology as an extremely energy-efficient computer logic device. In addition, the creation of C18 opens the way for the synthesis of other carbon allotropes, but for now, an in-depth study of the properties of the new molecule remains.
Conclusion
Carbon is the substance with the largest number of allotropic modifications. The project considers allotropic modifications of diamond, graphite, and fullerene. Carbon and its allotropic modifications are of great practical importance in human life and industry.
Most of the things in our lives we received thanks to allotropic modifications, for example, a drill, black graphite pencils and batteries.
Thanks to this project, we learned how and who helped us improve our lives.
Based on all the work done, we can draw the following conclusions:
- Firstly, I studied and examined in detail the types of allotropy.
- Secondly, I found out who discovered allotropy and where it is used.
- Thirdly, I made a stand that clearly shows what allotropy looks like.
Allotropic transformations and transitions
Allotropic transformations are most typical for nonmetallic substances (except for halogens (chlorine, bromine, iodine) and inert gases (argon, xenon and neon)), semimetals and, most rarely, for metals. Transitions of an element into a different form, different from its standard modification, occur when environmental conditions change. The main factors influencing allotropic transformations are changes in pressure and temperature, which can affect the elements both individually and in combination. Examples of elements with allotropy and their modifications are:
- carbon, which can be called the leader in the number of allotropic forms studied;
- phosphorus, for which 11 allotropes are known;
- oxygen, existing in the form of O2 (actually oxygen) and O3 (ozone);
- iron, which forms four crystalline modifications (α-Fe or ferrite, β-Fe, γ-Fe or austenite and δ-Fe);
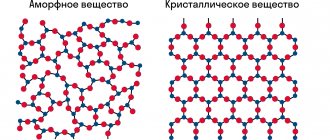
- silicon, which can be in both amorphous and crystalline forms;
- nitrogen, which has a polymer modification, is five times more powerful than non-nuclear explosive materials.
Any change can be reversible (that is, when returning to normal conditions, the substance goes into its standard form), enantiotropic, or irreversible, monotropic. Enantiotropic transitions include the transformation of sulfur from orthorhombic to monoclinic or the transition of white plastic tin (beta tin) to white brittle (gamma tin). Monotropic modification occurs, for example, when white phosphorus is modified into black.