The diamond mineral is essentially one of the many modifications of carbon. The physical properties of diamond are determined by the internal structure of the crystal.
Like other minerals, the physical properties of a diamond are assessed according to the following criteria:
- Hardness
- Density (specific gravity)
- Refractive index and dispersion
- Crystal lattice structure (see Chemical properties of diamonds)
A little history
There are many legends and beliefs associated with this amazing stone. And one of them is that a diamond brings good luck to its owner. But only winners in life can own it. And famous people who wore it include Napoleon Bonaparte, Julius Caesar and Holy Roman Emperor Louis the Fourth.
Europe recognized the diamond around the 5th-6th century BC. e., but only 550 years ago it gained its incredible popularity. After all, it was then that they learned how to cut it correctly in order to maximize the properties of a diamond. And all because of its incredible property - enormous strength, while the density of diamond is 3500 kg/m3. What other known mineral can boast the same characteristics?
But the fact that many consider a diamond to be a mineral that cannot be broken has led to the loss of rare and beautiful stones. For example, in 1476, during the war between Duke Charles the Bold (one of the first owners of brilliant-cut diamonds) and King Louis XI, the king's mercenaries managed to break into a tent standing on the battlefield. They were amazed by the placers of diamonds located there. They decided to check the authenticity of the stones with a hammer, and a large number of expensive and beautiful stones were turned into dust.
Diamonds became “friends of girls” only in the middle of the fifteenth century, thanks to Agnes Sorel, the favorite of Charles the Seventh. Now you know the name of the one who made many men “unhappy.”
Formation and origin of diamonds:
Most natural diamonds are between 1 billion and 3.5 billion years old. Many of them were formed at depths of 150 to 250 kilometers in the Earth's mantle, although some formed at depths of about 800 kilometers.
Under high pressure and temperature, carbon-containing liquids dissolved the minerals in the rock and replaced them with diamonds.
Diamonds were formed from this liquid either by reduction of oxidized carbon (such as CO2 or CO3) or by oxidation of a reduced phase such as methane.
Much later (tens - hundreds of millions of years ago) they were brought to the surface as a result of volcanic eruptions and deposited in igneous rocks known as kimberlites and lamproites.
Physical properties
An ignorant person, holding a diamond in his hands, is unlikely to guess what treasure he got. The unprocessed crystal looks very simple and inconspicuous. And most often the mineral is found in nature in the form of irregularly shaped fragments. Well, transparent, well, with high light refraction, which varies from 2.417 to 2.419. What's special about it? Only a sample of an octahedral shape (two pyramids connected by a base) can attract the attention of the average person by the play of light on its edges. It is the high refraction of light that causes what we later call brilliant shine; there is no birefringence. When exposed to the sun for a long time, most stones begin to glow in the dark.
Diamond is also known for its incredible hardness - 10 out of 10 on the Mohs scale. In other words, it is the hardest mineral known on earth. But what the density of a diamond is can be easily found in a reference book. But before we look, try to guess what it should be? Based on its hardness, it is quite high. But even here the diamond shows its paradox.
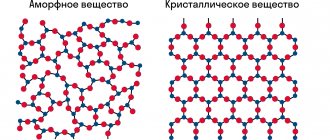
High hardness is due to the special structure of the cubic crystal lattice, where there is a carbon atom in each corner. One more atom is placed in the center of the face, and 4 atoms are located inside the cube. Thus, those atoms located in the center of the face are common to two neighboring cells, and those at the vertices are common to eight. This method of packing an atom is the densest.
The crystal splits to form smooth parallels (the so-called perfect cleavage). The fracture varies from conchoidal to splintered (of course, not along the cleavage).
Let's look at the reference book: the average density of diamond is 3500 kg/cub.m. Can vary from 3.47 to 4.55 grams per cubic centimeter. Not much for such a hard mineral. According to Razival, the grinding hardness is 140000.0.
Diamond hardness
According to the Mohs scale, the hardness of diamond is maximum and is equal to 10.
This generally accepted Mohs scale gives relative values for hardness. Its indicators indicate that a mineral with a higher number scratches a mineral with a lower one.
Next after diamond in terms of hardness on the scale is
corundum with a value of 9 . But its absolute hardness value is 150 times less than that of diamond - which indicates the absolute leadership of diamond in this regard.
There are other methods for determining hardness, but Mohs testing (scratching a mineral with another reference mineral) has proven to be the simplest and least destructive method, which is still widely used today.
Solid means it won't break?
The hardness of a diamond is not the same in different directions of the crystal. This is the basis for sawing, cutting and polishing diamonds. High hardness makes diamond exceptionally resistant to abrasion. Along with its hardness, diamond is quite brittle, which somewhat limits its use. Under the influence of a strong blow, a diamond easily splits along planes parallel to the faces of a regular octahedron.
Color spectrum
Another physical property that I would like to mention is the color of the stone. And color has a significant impact on the density of a diamond. The most common ones are colorless or yellowish, some have a bluish or brown tint. Colored crystals are much less common in nature, but the color variations are very diverse: pink and red, orange and bright yellow, green and blue, purple and cognac, cherry, gray and even black. Another name for colored diamonds is fancy. Although the most expensive ones were and remain transparent, colorless or with a bluish tint, the demand for stones of rare shades is growing, which means their price is also rising.
In addition, we are accustomed to the fact that diamonds are transparent, but there are also opaque ones. Color and transparency directly depend on the chemical composition of the crystals. Another pattern has been noticed: the darker it is, the lower the density of the diamond (g/cm3).
What is a diamond
Having a special structure, diamond is incredibly durable. The crystal lattice of the mineral has the shape of a cube, inside and on the tops of which carbon atoms are located. The presence of a strong bond between these atoms determines the structure of the gemstone: its hardness.
The chemical formula of diamond is extremely simple. It consists almost entirely of carbon. Therefore, the formula of diamond is C (carbon). The proportion of other elements in the composition of the mineral is insignificant (therefore, these elements are not taken into account in the formula). In general, carbon is quite small in nature (about 0.15% of the total number of elements).
The variety of applications of natural raw materials is due to the unique characteristics of the mineral. Common uses of gemstones include:
- in the manufacture of jewelry;
- in electronics: to avoid overheating of devices;
- in the manufacture of medical instruments.
The composition and physical properties of diamond also determine the use of the mineral in the field of telecommunications. Such raw materials are highly valued for their ability to withstand sudden changes in temperature and stress.
Note! The chemical composition of diamond also determines the use of natural raw materials in the mining industry: to increase the efficiency of the drill bit.
Only 15% of stones mined in the world are subsequently used to produce diamonds from natural raw materials. 45% of minerals are “conditionally suitable” for cutting minerals. The remaining raw materials are used for production and industrial needs.
Chemical composition and properties
As already mentioned, the mineral consists of 96.0–99.8% carbon, the atoms of which are interconnected in a cubic lattice. In addition, other chemical substances are found in the crystal - oxygen, nitrogen, boron and silicon, aluminum and manganese, iron and copper, titanium and zinc, nickel, etc. Possible inclusions of olivitate and chromite, graphite and pyrope, enstatine and others.
Quite often you can find crystals containing water and carbonic acid, carbon dioxide and other substances in a gaseous state. Most often, impurities are located closer to the periphery of the crystal.
As for chemical properties, diamond is very resistant to acids and alkalis, is not wetted by water, but is easily covered with a fatty film, even from ordinary hand touch. This property is used to identify a real stone. The mineral remains chemically inert until it is exposed to high temperature.
Diamond burns at a temperature of 850 °C, producing carbon dioxide. And when heated without air access by more than 1000 °C, it turns into an allotropic modification - graphite.
Specific Gravity of Gemstones (Density Table)
Gemstone specific gravity is a designation for the relative density of a piece of jewelry or gemstone.
Every jeweler knows from experience that some stones are “by weight” heavier than others. For example, colorless zircon weighs more than a diamond of the same size, and sapphire weighs more than an emerald. Scientists have long learned to express this quality quantitatively, and this plays a big role in recognizing substances. Water was used as a standard, and the weight of each substance was compared with the weight of an equal volume of pure water. The number obtained as a result of this comparison is called the specific gravity, or relative density of the substance. Thus, the specific gravity of a body is the ratio of its weight to the weight of pure water of equal volume. To obtain accurate data, water at a temperature of 4°C is used as a standard.
Table of specific gravity of precious stones and jewelry materials
The reference table provides specific gravity (or relative density) values for over 100 gemstones and jewelry materials.
| Gemstone | Specific gravity | Gemstone | Specific gravity |
| Aquamarine | 2,69 | Nephritis | 2,96 |
| Axinite | 3,28 | Obsidian | 2,35 |
| Diamond | 3,52 | Fire opal | 2,00 |
| Almandine | 4,2 | Opal | 2,1 |
| Amazonite | 2,56 | Orthoclase | 2,56 |
| Amblygonitis | 3,03 | Palladium | 11,3 |
| Anataz | 3,88 | turtle shell | 1,30 |
| Andalusite | 3,15 | Painite | 4,01 |
| Apatite | 3,21 | Periclase (synthetic) | 3,59 |
| Aragonite | 2,94 | Petalite | 2,39 |
| Bakelite | 1,26 | Pyrite | 4,9 |
| Barite | 4,5 | Pyrope | 3,7 |
| Benitoite | 3,67 | Platinum | 21,5 |
| Beryl (yellow) | 2,69 | Pleonastus | 3,8 |
| Beryllonite | 2,82 | Pollucite | 2,92 |
| Turquoise | 2,8 | Prehnite | 2,87 |
| Bowenite | 2,6 | Pseudofit | 2,7 |
| Brazilianite | 2,99 | Vegetable bone | 1,40 |
| Variscite | 2,55 | (yellow) | 3,56 |
| Vesuvian | 3,38 | Rodicite | 3,40 |
| Verdit | 2,9 | Rhodonite | 3,6 |
| Willemite | 4,03 | Rhodochrosite | 3,6 |
| Hambergite | 2,35 | Rutile (synthetic) | 4,25 |
| Ganospinel | Up to 3.97 | Silver | 10,5 |
| YYY | 7,05 | Singalit | 3,48 |
| Hematite | 5,05 | Scapolite yellow | 2,70 |
| Hessonite | 3,65 | Scapolite pink | 2,63 |
| Grossular | 3,5 | Ivory | 1,8 |
| Danburite | 3,00 | Smithsonite | 4,35 |
| Datolite | 2,95 | Sodalite | 2,3 |
| Demantoid | 3,85 | Sun stone | 2,64 |
| Diopside | 3,29 | Spessartine | 4,16 |
| Dioptase | 3,30 | Spodumene | 3,18 |
| Jade | 3,33 | Staurolite | 3,70 |
| Pearls (cultured) | 2,75 | Sphalerite | 4,09 |
| Pearl (oriental) | 2,71 | Sphene | 3,53 |
| Pearl pink | 2,85 | Talc | 2,75 |
| Gold 375 Ave. | 11,4 | Taffeite | 3,61 |
| Gold 583 Ave. | 13,93 | Strontium titanate | 5,13 |
| Gold 750 pr. | 15,4 | Topaz (white) | 3,56 |
| Gold 916 Ave. | 17,7 | Topaz (yellow) | 3,53 |
| Pure gold | 19,3 | Tourmaline | 3,06 |
| Emerald | 2,71 | Phenakite | 2,96 |
| Emerald (synthetic) | 2,65 | Fibrolite | 3,25 |
| Iolite | 2,59 | Fluorite | 3,18 |
| Calcite | 2,71 | Chalcedony | 2,6 |
| Carborundum | 3,17 | Chrysoberyl | 3,71 |
| Cassiterite | 6,9 | Chrysocolla | 2,20 |
| Quartz | 2,65 | Chrysolite | 3,34 |
| Quartz glass | 2,21 | Celluloid | 1,38 |
| Kyanite | 3,68 | Zircon (blue and colorless) | 4,69 |
| dug | 1,06 | Zircon (green) | 4-4,5 |
| Coral | 2,68 | Zoisite | 3,1 |
| Cornerupin | 3,32 | Zoisite (blue) | 3,35 |
| Corundum | 3,99 | Sheelit | 6,0 |
| Bone | 2,0 | Spinel | 3,60 |
| Cubic zirconia | 5,6-5,9 | Spinel (synthetic) | 3,63 |
| Labrador | 2,70 | Euclase | 3,10 |
| Lazulite | 3,09 | Ekanite | 3,28 |
| Lapis lazuli | 2,8 | Enstatite | 3,27 |
| Leucite | 2,47 | Epidote | 3,45 |
| Moon rock | 2,57 | Erinoid | 1,33 |
| Malachite | 3,8 | Amber | 1,08 |
| Modzavit | 2,35 | ||
| Note. When the specific gravity value varies by more than one or two units in the second decimal place, only one decimal place is given. | |||
____________
A source of information:
1. AndersonB. Definition of precious stones: Transl. from English /-Moscow, World of Stone 1996
2. DICTIONARY OF GEOLOGICAL TERMS AND CONCEPTS. / - Tomsk: 1996.
What else does the cost depend on?
The price range for diamonds is very wide, and the cost depends on many characteristics. But in any case, the price is always indicated per carat (0.2 g):
- Cut: The most highly rated of these is the 57 facet cut, or is also called the Tolkowsky cut. For small diamonds - 17 and 33. The remaining cuts are considered fancy, and their price is much lower. But still, we list other types of cuts: “Baryon”, “Quadrillion”, “Princess”, “Marquise”, “Rose”, “Briolet”, “Pear”, “Oval”, “Heart”, “Asscher”, “Emerald” ", "Radiant", "Triliant".
- Transparency: if the transparency of a diamond is ideal, there are no cracks, including microinclusions, then the price instantly increases by an order of magnitude, or even more.
- Stone size: here we are not talking about carats, so a diamond weighing one carat in diameter can be 6.5 mm, and if other indicators are as high, then the cost can be 10-12 thousand dollars per carat.
- Color. It all depends on fashion trends and the whims of the client. But the most valuable are still considered colorless and with a bluish tint.
Diamond cut:
The main types of diamond cuts are:
– round (with a standard number of 57 edges),
– fancy, which includes such types of cuts as “oval”, “pear”, “marquise”, “princess”, “radiant”, “heart”, “square”, “emerald”, “triangle” and other types.
The shape of a diamond's cut depends on the shape of the original diamond crystal .
Application
Having studied the properties of diamond, we can safely say: the stone is simply unique. But it is now used not only in jewelry. Science and industry take part of the world's stone reserves for their own needs. They only use small or defective stones.
What properties are valued by science and industry:
- high thermal conductivity;
- hardness;
- transparency (the ability to transmit UV and IR rays);
- crystal structure (can be a conductor, insulator). It can withstand high voltage and sudden temperature changes.
Medicine did not stand aside either, using diamonds in surgery. Scalpels are now being produced with diamond blades. Sharpening these blades makes the cuts super-fine. In laser machines, wounds are cauterized using diamonds. In laboratories with hazardous chemicals, diamond windows are installed.
Construction and repair tools, both for household use and professional ones - saws, metal knives, cutters and glass cutters, grinding wheels and much more - are coated with diamond chips to increase their service life. The tunnels are laid using a so-called roadheader. His knives are covered with a layer of diamond chips.
Refractive index and dispersion of diamond
The characteristic brilliance and “fire” of cut and polished diamonds is due to a very high refractive ( from 2.417 to 2.421 ) and strong dispersion ( 0.0574 ).
For reference: light refraction is the deviation of the direction of a light ray when entering another medium, where the light abruptly changes its speed. Dispersion is the difference in refractive index depending on the color (wavelength) of the light used.
Dispersion is the basis of the internal “fire” of diamonds.
Fig. 1: Schematic representation of dispersion:
Fig. 2: The play of “fire” in a diamond
Diamond has a refractive index of 2.42, which is the highest of any gemstone used in jewelry. That is why we have the pleasure of observing such properties of a diamond stone as sparkling, diamond shine .
The unique combination of dispersion with high refraction and the hardness of the diamond, which allows its edges to be polished without the slightest flaws, is exactly the unique set of properties that allowed the diamond to occupy the top in the world of jewelry.
Other physical properties of diamonds
Diamond is characterized by abnormally high thermal conductivity , which is 900–2300 W/(m K) and is the highest among all solids. This property allows us to consider diamond as a promising semiconductor (of course, provided that sufficiently cheap methods for producing synthetic diamonds are developed). Current silicon semiconductors can operate at temperatures up to 100°C, while diamond chips will operate at much higher temperatures.
Among other properties, it can be noted that diamond does not dissolve in acids and alkalis , is a dielectric , and has a very low coefficient of friction for metal in air (0.1), which is explained by the formation of thin films of adsorbed gas on the surface of the diamond, which play the role of a kind of lubricant. Under the influence of daylight and especially ultraviolet rays, most diamonds begin to glow blue, yellow and green; under the influence of cathode rays, luminescence appears in a pale blue color; under the influence of X-rays, it appears bluish. Diamonds have the property of sticking to certain fat mixtures. This property is widely used to extract diamonds in processing plants.
The melting point of diamond is 3700–4000 °C at a pressure of 11 GPa. In air, diamond begins to burn at 850°C. In a stream of pure oxygen it burns with a weak blue flame at 720–800 °C, completely turning into carbon dioxide. Heating diamond without access to air leads to its partial transformation into graphite at temperatures above 1500°C. When heated to 2000 °C without air access, diamond turns into graphite in 15-30 minutes.
ALL STONES - CATALOG | DIAMONDS (DIAMONDS) - CATALOG
Chemical composition of diamond | Diamond crystal structure | What types of diamonds are there? Diamond Crystal Shapes | What color are diamonds? | What kind of diamonds are in black diamond jewelry? | The largest diamond in the world
Share this article with your friends
Works by designers from the JEWELIRUM catalog
- Co.Cos Jewelry
- Co.Cos Jewelry
- Taiga Jewelry, Tomsk
- Taiga Jewelry, Tomsk
- Ilya Maksimov, Crimea
- Ilya Maksimov, Crimea
- UBIRING
- UBIRING
- Diamonds are Forever
- Diamonds are Forever
- Rings in natural style, Sergacheva Jewelery
- Earrings with pearls, Sergacheva Jewelery
- Cabochon ring, Minty Sky
- Fly earrings, Minty Sky
- Ring, Precious Park
- Ring, Precious Park
- Snake skin ring, Stoyanova Jewelery
- Chain earrings, Stoyanova Jewelery
- Children's pendant - stick, Matthew&Daniel
- Pendant for a child, Matthew&Daniel
- Bracelet, Svetlana Subbotina
- Ring with Slavic symbols, Svetlana Subbotina
- Indian style ring, Anna Goffman
- Indian style ring, Anna Goffman
- Earrings, ISTA
- Geometric ring, ISTA
- Earrings with enamel, PNJewelry
- Ring with enamel, PNJewelry
- Ring, Khramtsova Jewelry
- Ring, Khramtsova Jewelry
- Wedding rings to order, obruchalki.com
- Wedding rings to order, obruchalki.com
- Earrings, Yuri Bylkov
- Earrings, Yuri Bylkov
- Titanium bracelets, LanaMuransky
- Titanium pendant, LanaMuransky
- Brooch Elephant (after Salvador Dali), THING
- Ring Veil, THING
- Mace earrings, VLADIMIR MARKIN
- Cufflinks, jewelry mechanics, VLADIMIR MARKIN
- Drop-shaped ring, EKATERINA TOLSTAYA
- Drop-shaped earrings, EKATERINA TOLSTAYA
- Necklace with amber, LETA
- Earrings with amber, LETA
- Children's earrings, combinable, FASHBY
- Children's earrings, combinable, FASHBY
- Ring of architectural form, Elizaveta Malafeevskaya MANU_L
- Architectural bracelet, Elizaveta Malafeevskaya MANU_L
- Set Ginkgo Leaf, SHABUT JEWELLERY
- Brooch Wearable porcelain, SHABUT JEWELLERY
- Architectural ring, GEOMETRY
- Brooch, porcelain, GEOMETRY
- Necklace made of polymer clay, LICORNE ART
- Brooch made of polymer clay, LICORNE ART
- Ring, avant-garde, VALERY SEREDIN
- Bracelet, avant-garde. VALERY SEREDIN
- Wooden set, Scandinavian/Japanese minimalism, VLADIMIR SHESTAKOV
- Ring, Scandinavian/Japanese minimalism, VLADIMIR SHESTAKOV
- Earrings, TON ANT
- Ring, TON ANT
- Architectural ring, ANCHOR
- Architectural necklace, ANCHOR
- Earrings, GOHFELD JEWELLERY
- Necklace, GOHFELD JEWELLERY
- Massive ring, YAKISCHIK
- Designer jewelry, YAKISCHIK
- Architectural ring, ONE DAY ART
- Architectural ring, ONE DAY ART
- Brooch, bionics, VALERIYA MARKOVA (TESSA)
- Unclosed ring, bionics, VALERIYA MARKOVA (TESSA)
- Ring, bionics, BEAVERS
- Earrings, bionics, BEAVERS
- Earrings, asymmetry, VAGANOVA JEWELRY
- Airplane ring, VAGANOVA JEWELRY
- Flower ring, ALCHEMIA JEWELLERY
- Set, ALCHEMIA JEWELLERY
- Pendant-cat, ethnic, STUDIO OF ILYA AND VERA PALKIN
- Earrings, STUDIO OF ILYA AND VERA PALKIN
In the school curriculum
Such a characteristic as diamond density is found even in the school curriculum. They study it in a subject such as physics, in the section “Fundamentals of molecular kinetic theory” in the 10th grade. And the problem is solved. It sounds like this in full:
There is a diamond whose density in kg/m3 is 3500. What volume will the atoms of the substance occupy in the amount of 1022? (Myakishev’s problem book). It turns out that the properties of diamonds are studied in school. And it’s not only in this problem book that similar problems can be found. It is also possible to write the condition like this:
- The density of diamond is 3500. What volume will 1022 of its molecules occupy?
Directory
Density of solids and liquids
| Substance | Density g/ml = 103 kg/m3 | Substance | Density g/ml = 103 kg/m3 |
| Agate | 2,6 | Cork | 0,25 |
| Alabaster | 1,8 | Mercury | 13,6 |
| Aluminum | 2,7 | Salo | 0,9 |
| Diamond | 3,5 | Lead | 11,3 |
| Asbestos | 2,4 | Silver | 10,3 |
| Asphalt | 1,4 | Turpentine | 0,85 |
| Acetone | 0,8 | Mica | 2,8 |
| Petrol | 0,7 | Resin (gum) | 1,1 |
| Borax | 1,7 | → black | 1,1 |
| Var | 1 | Denatured alcohol | 0,8 |
| Sea water | 1,03 | → ethyl | 0,8 |
| Wax (laboratory) | 1 | Alcohol | 0,79 |
| → bee | 0,95 | Soft steel | 7,9 |
| Germanium | 5,4 | → carbonaceous (<1% C) | 7,8 |
| Glycerol | 1,3 | Sealing wax | 1,8 |
| Granite | 2,7 | Alloys | |
| Graphite | 2,3 | → Alni | 6,9 |
| Dry wood | → Alnico | 7,1 | |
| → Backout | 1,3 | → Babbitt (80% Sn) | 7,3 |
| → Balsa (cork) | 0,2 | → Aluminum bronze (8% Al) | 7,7 |
| → Bamboo | 0,4 | → → phosphorous | 8,9 |
| → Beech | 0,75 | → Duralumin | 2,8 |
| → Oak | 0,7 | → Stainless iron (12% Cr) | 7,7 |
| → Cedar | 0,55 | → Mirror bronze | 8,4 |
| → Mahogany | 0,8 | → Invar | 8 |
| → Boxwood | 1 | → Inconel | 8,5 |
| → Pine (white) | 0,5 | → Constantan | 8,9 |
| → Teak wood | 0,85 | → Cronit | 8,1 |
| → Ebony | 1,2 | → Brass (60/40) | 8,4 |
| Gelatin | 1,3 | → → (70/30) | 8,5 |
| Silicon iron | 6,9 | → Lo-Ex | 2,7 |
| → welding | 7,8 | → Magnalium | 2,6 |
| Ash (wood) | 0,75 | → Mazak (No. 2) | 6,7 |
| Gold (22 carat) | 17,5 | → Manganin | 8,5 |
| → (9 carats) | 11,3 | → Beryllium copper | 8,2 |
| Tungsten Carbide (6% CO) | 15 | → Monel | 8,8 |
| → tungsten (12% CO) | 14,2 | → Mu-metal | 8,8 |
| Crystalline quartz | 2,6 | → German silver | 8,4 |
| → melted translucent | 2,1 | Nickel-silver | 8,8 |
| → → transparent | 2,2 | Nickel-chrome | 8,4 |
| Quartz sand (pure) | 2,6 | Nikonik | 8,2 |
| Keramot | 1,6 | Permalloy | 8,6 |
| Kerosene | 0,8 | Platinum-iridium (90/10) | 21,5 |
| Kaolin | 2,6 | Prina soft (70% Sn, 30% Pb) | 8,3 |
| Corundum | 4 | Alloy "Y" | 2,8 |
| Bone | 1,9 | Supermalloy | 8,9 |
| → ivory | 1,8 | Gunmetal | 8,2 |
| Silicon | 2,4 | Elinvar | 8,1 |
| Xylene | 0,85 | Thiokol | 1,4 |
| Ice | 0,92 | Coal (anthracite) | 1,6 |
| Animal oil | 0,9 | → (bituminous) | 1,4 |
| → castor | 0,95 | → (woody) | 0,4 |
| → flaxseed | 0,95 | → (retort) | 1,9 |
| → olive | 0,9 | White spirit | 0,85 |
| → paraffin | 0,8 | Porcelain | 2,3 |
| Copper | 8,9 | Chromium | 7,2 |
| Micalex | 2,4 | Cast iron | 7 |
| Milk | 1,03 | Slate | 2,8 |
| Marble | 2,7 | Ebonite | 1,2 |
| Emery | 4 | Amber | 1,1 |
| Oil | 0,8 | ||
| Nichrome | 8,4 | ||
| Tin | 7,3 | ||
| Paraffin | 0,9 | ||
| Sand (dry) | 1,6 | ||
Density of gases and vapors
| Substance | Formula | Density g/ml = 103 kg/m3 | Substance | Formula | Density g/ml = 103 kg/m3 |
| Nitrogen | N2 | 1.2505 | Neon | Ne | 0.8999 |
| Ammonia | NH3 | 0.7714 | Nitrosyl | ||
| Argon | Ar | 1.7839 | → fluoride | NOF | 2.176* |
| Acetylene | C2H2 | 1.1709 | → chloride | NOCl | 2.992 |
| Boron fluoride | BF3 | 2.99 | Ozone | O3 | 2.22 |
| n-Butane | C4H10 | 2.703 | Nitric oxide | NO | 1.3402 |
| i-Butane | C4H10 | 2.673 | Propane | C3H8 | 2.0037 |
| Hydrogen | H2 | 0.08987 | Propylene | C3H6 | 1.915 |
| → bromide | HBr | 3.664 | Radon | Rn | 9.73 |
| → iodide | Hl | 5.789 | Sulfur | ||
| → arsenic | H3As | 3.48 | → dioxide | SO2 | 2.9263 |
| → selenium | H2Se | 3.6643 | → hexafluoride | SF6 | 6.50* |
| → sulfurous | H2S | 1.5392 | Silan | ||
| → telluride | H2Te | 5.81 | → dimethyl | SiH2(CH3)2 | 2.73 |
| → phosphorous | H3P | 1.53 | → methyl | SiH3CH3 | 2.08 |
| → chloride | HCl | 1.6391 | → chloride | SiH3Cl | 3.03 |
| Air | — | 1.2928 | → trifluoride | SiHF3 | 3.89 |
| Helium | He | 0.1785 | Stibine (15°C, 754 mmHg) | SbH3 | 5.3 |
| Germany tetrahydride | GeH4 | 3.42 | Sulfuryl fluoride | SO2F2 | 3.72* |
| Dimethyl sulfide | C2H6S | 0.848* | Trimethylamine | (CH3)3N | 2.580* |
| Dimethyl disulfide | (CH3S)2 | 1.062* | Trimethylboron | (CH3)3B | 2.52 |
| Dimethylamine | (CH3)2NH | 1.966* | Carbon | ||
| Difluorodichloromethane | CF2Cl2 | 5.51 | → dioxide | CO2 | 1.9768 |
| Dician | C2N2 | 2.335* | → oxide | CO | 1.25 |
| Nitrous oxide | N2O | 1.978 | → sulfur dioxide | COS | 2.72 |
| Oxygen | O2 | 1.42904 | Phosphorus | ||
| Silicon | → fluoride | PF3 | 3.907* | ||
| → fluoride | SiF4 | 4.9605 | → oxyfluoride | POF3 | 4.8 |
| → hexahydride | Si2H6 | 2.85 | → pentafluoride | PF5 | 5.81 |
| → tetrahydride | SiH4 | 1.44 | Fluorine | F2 | 1.695 |
| Krypton | Kr | 3.74 | Nitrogen fluoride | NO2F | 2.9 |
| Xenon | Xe | 5.89 | Chlorine | Cl2 | 3.22 |
| Methane | CH4 | 0.7168 | → dioxide | ClO2 | 3.09* |
| Methylene chloride | CH3Cl | 2.307 | → oxide | Cl2O | 3.89* |
| Methylamine | CH5N | 1.388 | Nitrogen oxychloride | NO2Cl | 2.57 |
| Methyl mercaptan | CH3SH | 0.87 | Ethane | C2H6 | 1.356 |
| Methyl ether | C2H6O | 2.1098 | Ethylene | C2H4 | 1.2605 |
| Methyl fluoride | CH3F | 1.545 | |||
| Methyl chloride | CH3Cl | 2.307 | |||
| Arsenic fluoride | AsF5 | 7.71 | |||
Note: Reference data is sourced from online publications and may not be considered "official" or "completely accurate." As a rule, Internet directories do not provide links to scientific works that are the basis of published data. We try to take information from the most reliable scientific sites. However, if anyone is interested in links to experiments, we advise you to conduct an in-depth search on the Internet yourself. We will be grateful for any comments on our reference tables, and especially for clarification of existing information or addition of reference data.
What do the stones look like?
There is a classification of minerals, according to which they distinguish:
- diamond. Such stones are shining specimens, suitably processed in the workshop;
- board. This is the name given to irregular fine-grained specimens. Natural stone is painted black;
- ballas. The spherical gems have a grayish cloudy structure. Therefore, ballas are classified as translucent crystals.
It is worth telling what other minerals are present in nature. Carbonado is a multi-colored crystals, colored gray or black. Such natural raw materials have a coarse-grained or dense structure.
Yakutite is also distinguished. According to its characteristics, the stone is a dark mineral with small inclusions and a silvery sheen on the surface.
Magic properties
Important! Like all natural minerals, they have a magical purpose. Diamond is a transparent stone, and accordingly, gives a pure aura to the owner. The stone can help in both love affairs and career.
All the advantages of the stone that people with magical powers attribute:
- provides a guarantee of protection against magical effects of any direction;
- bestows courage, self-confidence, fortitude;
- protects against bad habits;
- makes the owner happy.
Diamond mixed with green shades gives a woman health and strong family ties.
For all its positive properties, the mineral is demanding of its owner. If a person has negative thoughts towards other people, then the stone will not work, this also applies to sinners. A diamond passed down by inheritance acquires a magical effect on the new owner only after 10 years of constant wear. According to legend, in order to acquire magical properties, a diamond must be given to a person, and not purchased independently. Under no circumstances should you steal a diamond or jewelry with it, this will bring misfortune and big life problems to the criminal.
In any case, a brilliant diamond is a beautiful piece of jewelry that will suit any person with a pure soul.
According to the zodiac system, it is better to choose a diamond for Aries and Libra. The stone bestows upon these signs the maximum positive qualities in the form of confidence, courage and determination. Pisces should absolutely not wear the stone, as it will not bring any action.
Blue diamonds are suitable for water signs, and red shades of the mineral are suitable for fire signs.
Subtleties of care
Despite the special structure of the diamond, diamond jewelry should be protected from shock and mechanical impact. At home, jewelry is regularly washed in warm soapy water. Diamond jewelry is left in this solution for 20 minutes. After the specified time, the products are cleaned with a soft cloth. After this, the jewelry is washed in cool, clean water.
Diamonds are stored in tightly closed boxes and velvet bags. At the same time, precious stones should not scratch each other.
There are various ways to obtain gems. A set of jewelry with expensive diamonds indicates a woman’s wealth and high status in society. Female representatives should learn everything about diamond in terms of its magical properties. For example, a diamond ring promotes the active development of creative potential.
Healing properties of the stone
According to ligotherapists, the mineral promotes the active renewal of body cells. In many teachings it is used as a rejuvenating agent.
With the help of the mineral they treat:
- nervous diseases;
- kidney pathologies;
- heart diseases.
In former times, mountain warriors wore rings decorated with diamonds. It was believed that such jewelry gave strength of spirit, made a person courageous and invincible. The gemstone protects against bad deeds. Such a talisman brings sincere happiness to the owner.