Graphite is a mineral from the class of native elements, one of the allotropic modifications of carbon. The mineral is widely distributed in nature. Usually found in the form of single flakes, plates and clusters, varying in size and graphite content. There are deposits of crystalline graphite associated with igneous rocks or crystalline shales, as well as cryptocrystalline graphite formed during the metamorphism of coals.
- Structure
- Property
- Morphology
- Source
- Application
- Classification
- Physical properties
- Optical properties
- Crystallographic properties
See also:
Agate - price and healing and magical properties
Physical properties and structure of diamond
STRUCTURE
A polymorphic (allotropic) hexagonal crystalline modification of pure carbon, the most stable under the conditions of the earth's crust. The layers of the crystal lattice can be arranged in different ways, forming many polytypes, with symmetry from the hexagonal system (hexagonal-dipyramidal symmetry) to trigonal (wc di-trigonal-scalenohedral). Stratified graphite crystal lattice. In layers, C atoms are located at the sites of hexagonal cells of the layer. Each C atom is surrounded by three neighbors at a distance of 1.42Α
There are two modifications of graphite: α-graphite (hexagonal P63/mmc) and β-graphite (rhombohedral R(-3)m). They differ in the packing of layers. In α-graphite, half of the atoms of each layer are located above and below the centers of the hexagon (set... ABABAB...), and in β-graphite, every fourth layer repeats the first. It is convenient to depict rhombohedral graphite in hexagonal axes to show its layered structure.
β-graphite is not observed in its pure form, since it is a metastable phase. However, in natural graphites the content of the rhombohedral phase can reach 30%. At a temperature of 2500-3300 K, rhombohedral graphite completely transforms into hexagonal graphite.
Therapeutic effect
Homeopaths were the first to appreciate graphite. They found that the mineral is suitable for the treatment of skin pathologies (eczema, psoriasis, lichen, etc.).
Today the list has been expanded:
- Metabolic disease.
- Malfunction of the thyroid gland.
- Respiratory tract diseases (rhinitis, bronchial asthma).
- Gastrointestinal problems (gastritis, gastric ulcer, duodenal ulcer, colitis).
- Women's ailments (amenorrhea, chronic inflammation of the ovaries, mastopathy).
- Conjunctivitis, cataracts, stye.
The mineral also “supervises” emotional health. It is prescribed for morning headaches, neurasthenia, apathy, and depression.
Sources
- https://vseprokamni.ru/vidy/organicheskie/ximicheskaya-formula-grafita.html
- https://FB.ru/article/351885/chto-takoe-grifel-iv-ch-m-zaklyuchayutsya-ego-unikalnyie-svoystva-dlya-risovaniya
- https://mineralpro.ru/minerals/graphite/
- https://www.syl.ru/article/307195/formula-grafita-allotropiya-ugleroda
- https://FB.ru/article/277065/grafit-plotnost-svoystva-osobennosti-primeneniya-i-vidyi
- https://vseprokamni.ru/svoistva/kristallicheskaja-reshetka-grafita.html
- https://crystal-wow.ru/kamni/fizicheskie-svojstva-grafita-tablica.html
- https://vseprokamni.ru/svoistva/fizicheskie-i-himicheskie-svojstva-granita.html
- https://natrukodel.ru/prochie/himicheskaya-formula-grafita
- https://abc-24.info/prostoj-karandash-istoriya/
- https://FB.ru/article/236131/allotropnyie-veschestva-almaz-i-grafit-formula-grafita-i-almaza
PROPERTIES
Conducts electricity well. Unlike diamond, it has a low hardness (1 on the Mohs scale). Relatively soft. Once exposed to high temperatures, it becomes slightly harder and becomes very brittle. Density 2.08-2.23 g/cm³. Color dark gray, metallic luster. Fusible, resistant to heat in the absence of air. Greasy (slippery) to the touch. Natural graphite contains 10-12% clays and iron oxides. When rubbed, it peels off in separate flakes (this property is used in pencils).
The thermal conductivity coefficient of graphite from 278.4 to 2435 W / (m * K) depends on the grade of graphite, on the direction relative to the basal planes and on temperature.
The electrical conductivity of graphite single crystals is anisotropic, in the direction parallel to the basal plane it is close to the metallic one, in the perpendicular direction it is hundreds of times lower. The minimum conductivity value is observed in the range of 300-1300 K, and the position of the minimum is shifted to the low-temperature region for ideal crystal structures. Recrystallized graphite has the highest electrical conductivity.
The coefficient of thermal expansion of graphite up to 700 K is negative in the direction of the basal planes (graphite contracts when heated), its absolute value decreases with increasing temperature. Above 700 K the coefficient of thermal expansion becomes positive. In the direction perpendicular to the basal planes, the coefficient of thermal expansion is positive, practically independent of temperature and more than 20 times higher than the absolute average value for the basal planes.
Graphite single crystals are diamagnetic, the magnetic susceptibility is negligible in the basal plane and high in orthogonal basal planes. The Hall coefficient changes from positive to negative at 2400 K.
Place of Birth
The largest deposits of graphite are located in China, Ukraine, Mexico, Canada and South Korea. Mineral deposits are economically beneficial for the country in which it is located. In the process of developing deposits, providing industry with the necessary raw materials. The stone formed clusters of gray color. Graphite ore is mined by open-pit mining, and lump mineral is mined by underground mining.
One of the high-temperature vein mineral deposits is the deposit in Ceylon, which is of great industrial importance. Here graphite veins are located among gneisses. There are similar deposits in Quebec, Montana and England.
Where and how is it mined?
There are commercial-scale graphite deposits on all continents:
- Both Americas - USA, Canada, Brazil;
- Europe – Germany, Greenland, Italy;
- Australia.
The raw materials of each graphite mine can be distinguished by structure, color, and other characteristics.
Russia has three largest deposits:
- Buryatia – high-quality densely crystalline raw materials.
- Krasnodar region (two) – dense, finely crystalline, scaly, graphite shales.
Graphites are formed by coal pyrolysis or under the influence of extremely high temperatures and pressure. For example, the outpouring of magma onto coal deposits.
It is mined by above-ground or underground methods. Graphite crystals are found in shales, marbles, and other organic rocks.
The annual global production of graphite is 600 thousand tons.
Reserves
World reserves of graphite (1978, thousand tons) in capitalist and developing countries: flake - South America, 136; Europe, 3500; Africa, 5442; Asia, 900; densely crystalline - Asia, 2900; cryptocrystalline - North America (without USA), 3084; Europe, 5623; Asia, 6168. For graphite mining, see Art. graphite industry.
MORPHOLOGY
Well-formed crystals are rare. The crystals are lamellar, scaly, curved, usually of an imperfect lamellar shape. Most often these are leaves without crystallographic outlines and their aggregates. Forms continuous radial-radial aggregates, cryptocrystalline, sheet or round, less often - spherulite aggregates of concentric-zonal structure. In coarse-crystalline sediments, triangular shading is often observed on the (0001) planes.
Position of carbon in the periodic table
Carbon is an element of the fourth group, the second period of the periodic table of chemical elements of D. I. Mendeleev. It is organogenic. This group also includes oxygen, nitrogen and hydrogen. This means that they are part of all living organisms on the planet, forming their basis.
This position determines the structure of the carbon atom. Its outermost energy level contains four electrons. This means that a given chemical element can exhibit both positive and negative oxidation states (+4 or -4).
ORIGIN
It forms at high temperatures in volcanic and igneous rocks, pegmatites and skarns. It occurs in quartz veins with wolframite and other minerals in mid-temperature hydrothermal polymetallic deposits. Common in metamorphic rocks - crystalline schists, gneisses, marbles. Large deposits are formed as a result of coal pyrolysis under the influence of traps in coal deposits (Tunguska basin). Accessory mineral of meteorites. Associated minerals: quartz, pyrite, garnets, spinel.
Description of graphite:
Graphite (translated from Greek as “I write”) is a natural material belonging to the class of native elements, an allotropic modification of carbon. The chemical formula of graphite is C.
Along with graphite and diamond, there are many more allotropic forms of carbon. For example, graphene, fullerene, carbon nanotubes, etc. The properties of these substances are completely different from each other.
Graphite is widely distributed in nature as a mineral. It is usually found in the form of individual scales, plates and clusters, varying in size and content.
There are deposits of crystalline graphite associated with igneous rocks or crystalline shales, and cryptocrystalline graphite formed during the metamorphism of coals.
Natural graphite is not pure in its chemical composition. It contains large quantities (up to 10-25%) of ash, consisting of various components (Fe2O3, SiO2, Al2O3, MgO, P2O5, CuO, CaO, etc.), gases (up to 2%) and bitumen, and sometimes water.
Graphite is also obtained artificially in various ways. For example, by heating a mixture of coke and pitch to 2,800 °C.
APPLICATION
For the manufacture of melting crucibles, lining plates - the application is based on the high heat resistance of graphite (in the absence of oxygen), its chemical resistance to a number of molten metals. It is used in electrodes and heating elements due to its high electrical conductivity and chemical resistance to almost all aggressive aqueous solutions (much higher than that of precious metals). For the production of chemically active metals by electrolysis of melts, solid lubricants, combined liquid and paste lubricants, plastic fillers.
it is a neutron moderator in nuclear reactors, a component of a composition for the production of rods with black graphite (mixed with kaolin). It is used to obtain synthetic diamonds as a nanometer length standard for the calibration of tunnel effect microscope and atomic force microscope scanners, for the manufacture of contact brushes and current collectors for various electrical machines, electric vehicles and trolley overhead cranes, high-power rheostats and other devices where required reliable mobile electrical contact for the manufacture of thermal protection for the nose of ballistic missile warheads and re-entry spacecraft.
Graphite - Before
| Molecular mass | 12.01 g/mol |
| origin of name | from ancient Greek. - write write |
| IMA status | valid, first described before 1959 (pre-IMA) |
Story
The history of the mineral graphite is quite confusing and difficult to study, since the mineral is very similar to other stones. However, mineralogists studying the history of stones still find some information about the history of the origin and use of graphite.
They believe that graphite stone began to be used in medieval times. Wherever the stone was found on the surface, it left clear greasy stains. When ancient people noticed this, they began to use the stone for writing and drawing. Later, thanks to this property, the stone began to be used in England. Using graphite crayons, English shepherds made marks on the wool of sheep so as not to lose sight of them.
The very first artists created crayons and similarities to modern pencils from graphite. They used them to paint graffiti-style paintings. Engineers and architects used graphite fragments to make drawings when designing various buildings, structures, and temples.
Some researchers have found the first mention of graphite 4000 BC. They claim that the mineral was used by the primitive people of the Boyan Morica culture to decorate pottery. Using graphite stone, they painted household items in grayish and black colors, and also painted various patterns on them.
The history of the name of the stone is associated with the famous mineralogist and chemist Abram Werner. In the process of compiling a physical and chemical description of graphite, the scientist took as a basis the property of the stone to leave a black color behind itself. This explains the ancient Greek name for graphite, meaning “to write, record.”
An interesting fact is that the name of the discoverer of carbon is still unknown to science, as is the fact itself, which of its forms - diamond, graphite - was discovered first. It is only known that carbon has 3 isotopes, two of which are stable, and one is radioactive and has a half-life of more than 5 thousand years. A study of various 18th century chemistry textbooks also does not provide a clear answer about carbon derivatives. Among the experiments of ancient chemists, combustion of various chemical elements and allotropic substances was often used. Thus, in one of the works there is a mention of the burning of diamond, where it was emphasized that this mineral is the only one that burns without a residue. The scientist Lavoisier, conducting similar experiments, came to the conclusion that diamond is a crystalline form of carbon. The second allotropic mineral of carbon (graphite) was long considered a modified form of lead and was called plumbago, until the chemist Guiton de Morveau carefully heated diamond, turning it into graphite, and then into carbonic acid.
PHYSICAL PROPERTIES
| Mineral color | iron black turns into steel gray |
| Line color | black becomes steel gray |
| Transparency | opaque |
| Shine | semi-metallic |
| Cleavage | very good on {0001} |
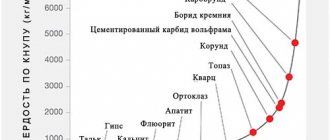
| Hardness (Mohs scale) | 1-2 |
| Break up | like mica |
| Power | flexible |
| Density (measured) | 2.09 - 2.23 g/cm3 |
| Radioactivity (GRApi) | 0 |
Where is it used?
The use of graphite is an integral part of some technological processes. Used in industries such as mechanical engineering, nuclear engineering, metallurgy, electrical engineering, and the chemical industry.
graphite electrodes
graphite bushings
When creating chemical equipment, certain varieties of graphite impregnated with various synthetic resins are used. This is caused by the property of graphite not to react with oxidizing agents.
graphite sealant for pumps
Mechanical seals, bearings, reactor vessels, and lining tiles are made from artificial graphite.
How are graphite rods produced?
The production process is automated and divided into several stages. First, the lead itself is produced from a mixture of clay and graphite. The hardness of the future pencil depends on the ratio of the proportions of these materials. The more graphite, the softer the pencil.
The clay is crushed and mixed with warm water, then liquid glass is added to remove impurities (sand). Next, graphite is added to the clay according to the recipe. This whole mass is mixed with a starch binder.
The core mass is brought to a certain temperature and consistency. At this stage of production, strict process rules must be followed, otherwise the raw materials will be spoiled. The resulting “dough” is passed through a press, in which the required product with three gaps is formed through special rollers. This process removes air bubbles. If this stage occurs in violation of technology, then the future pencil will crumble when pressed.
Next, the mass takes on the desired shape and is glued between two planks. The result is a familiar pencil to all of us.
What chemical bonds are the strongest in a crystal?
Covalent bonds (where electrons belong to two atoms at the same time) are more common and usually stronger. Thus, in diamond, carbon atoms are connected by covalent bonds to their nearest neighbors in four directions, which is why the crystal is so hard.
Interesting materials:
How to transfer all contacts from your phone to an LG SIM card? How to transfer everything to a new phone? How to transfer all numbers from SIM card to phone? How to re-glue protective glass on your phone? How to re-glue the protective glass on your phone? How to change channels on TV using your phone? How to switch between Facebook accounts on your phone? How to switch your phone screen to your TV screen? How to switch language on your phone? How to switch the language on a Samsung phone?