Graphite
- a mineral from the class of native elements, one of the allotropic modifications of carbon. A common mineral in nature. It is usually found in the form of individual flakes, plates and clusters, varying in size and graphite content. There are deposits of crystalline graphite associated with igneous rocks or crystalline shales, and cryptocrystalline graphite formed during the metamorphism of coals.
- Structure
- Properties
- Morphology
- Origin
- Application
- Classification
- Physical properties
- Optical properties
- Crystallographic properties
See also:
Agate
– price and medicinal, magical properties
Physical properties and structure of diamond
STRUCTURE
Hexagonal crystalline polymorphic (allotropic) modification of pure carbon, the most stable under the conditions of the earth's crust. The layers of the crystal lattice can be differently positioned relative to each other, forming a whole range of polytypes, with symmetry from hexagonal system (dihexagonal-dipyramidal type of symmetry) to trigonal (ditrigonal-scalenohedral symmetry). The crystal lattice of graphite is layered. In layers, C atoms are located at the sites of hexagonal cells of the layer. Each C atom is surrounded by three neighboring ones with a distance of 1.42Α
There are two modifications of graphite: α-graphite (hexagonal P63/mmc) and β-graphite (rhombohedral R(-3)m). They differ in the packing of layers. In α-graphite, half of the atoms of each layer are located above and below the centers of the hexagon (laying...AVAVAVA...), and in β-graphite, every fourth layer repeats the first. It is convenient to represent rhombohedral graphite along hexagonal axes to show its layered structure.
β-graphite is not observed in its pure form, since it is a metastable phase. However, in natural graphites the content of the rhombohedral phase can reach 30%. At a temperature of 2500-3300 K, rhombohedral graphite completely transforms into hexagonal graphite.
Recycling
Industrial processing of graphite makes it possible to obtain not only different grades of graphite, but also finished graphite products. Commercial types of this mineral are produced by starting the beneficiation process of graphite-containing ores. Based on the degree of purification, the resulting graphite concentrate will be classified into grades used in industry, as well as by areas of use.
Processing into thermally expanded graphite
First, crystalline graphite undergoes an oxidation process. This process involves the introduction of molecules and ions of nitric or sulfuric acid into the interlayer space of the crystal lattice of the mineral being processed. After oxidation, the graphite undergoes washing and subsequent drying to remove residual water. At the next stage, the mineral undergoes heat treatment at a temperature of 1000 degrees and at a high heating rate. Due to the very rapid heating of the mineral, the process of gas release and decomposition of sulfuric acid molecules embedded in the interlayer space of the crystal lattice of the mineral is triggered.
The release of gaseous substances helps to create an excess disjoining pressure of 400 atmospheres in the intercrystalline space. As a result, thermally expanded graphite is formed, which has a high specific surface area and low bulk density. If sulfuric acid is used to create this artificial mineral, a certain residual amount of sulfur remains in the finished material. At the next stage, the finished thermally expanded graphite is rolled, in some cases additionally reinforced and pressed with the addition of special additives to obtain finished products.
To obtain various grades of artificial graphite
In the process of producing synthetic graphite, petroleum coke is often used, which acts as a structural filler, as well as coal tar pitch, which is used as a binder. In structural types of artificial minerals, soot and graphite of natural origin are used as a special additive. Also, various artificial resins (furan, phenolic, and so on) can be used as a binding component instead of pitch.
The synthetic graphite production process consists of the following technological industrial stages:
- coke is prepared for the production process, it undergoes preliminary crushing by crushing, pierced, and also dispersed into separate fractions;
- the binder is prepared;
- a carbonaceous mass is prepared;
- green unfired blanks are formed into a solid matrix;
- the blanks are fired;
- the process of graphitization of previously fired workpieces is carried out;
- At the final stage, the blanks undergo mechanical processing to obtain the shape of the finished product.
To obtain composite materials
Today, modern industry produces anti-friction carbon materials of such brands as:
- baked antifriction materials of the AO brand;
- graphite and antifriction materials of the AG brand;
- materials with babbitt, tin and lead impregnation;
- graphite plastic materials of the AFGM, AFG-80VS, 7V-2A, KV, KM, AMS brands.
Such materials are made from unpleasant coke that does not undergo a calcination procedure, as well as coal pitch with the addition of natural graphite. In order to increase the density of the material, the technique of impregnation with metals is used.
PROPERTIES
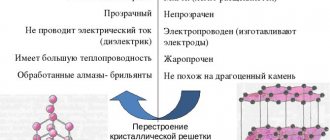
Conducts electricity well. Unlike diamond, it has low hardness (1 on the Mohs scale). Relatively soft. After exposure to high temperatures it becomes slightly harder and becomes very brittle. Density 2.08-2.23 g/cm³. The color is dark gray, metallic luster. Infusible, stable when heated in the absence of air. Greasy (slippery) to the touch. Natural graphite contains 10-12% admixtures of clays and iron oxides. When rubbed, it separates into separate flakes (this property is used in pencils).
The thermal conductivity of graphite is from 278.4 to 2435 W/(m*K), depending on the grade of graphite, on the direction relative to the basal planes and on temperature.
The electrical conductivity of graphite single crystals is anisotropic, in the direction parallel to the basal plane it is close to the metallic one, in the perpendicular direction it is hundreds of times less. The minimum conductivity value is observed in the range of 300–1300 K, and the position of the minimum shifts to the region of low temperatures for perfect crystalline structures. Recrystallized graphite has the highest electrical conductivity.
The coefficient of thermal expansion of graphite up to 700 K is negative in the direction of the basal planes (graphite contracts when heated), its absolute value decreases with increasing temperature. Above 700 K the coefficient of thermal expansion becomes positive. In the direction perpendicular to the basal planes, the coefficient of thermal expansion is positive, practically independent of temperature and more than 20 times higher than the average absolute value for the basal planes.
Graphite single crystals are diamagnetic; the magnetic susceptibility is insignificant in the basal plane and high in planes orthogonal to the basal planes. The Hall coefficient changes from positive to negative at 2400 K.
Melting temperature
The range of temperatures at which graphite can melt is very diverse. Much depends, for example, on the final objectives of the operation. The temperature range is also determined by external conditions, the characteristics of the composition of a particular mineral, and the use of additional means of influencing graphite during heat treatment. The melting temperature at which it is possible to obtain graphite ready for use varies from 2600 to 3800 °C. Calculation on the Kelvin scale is also practiced. In this case, it already reaches 4000° K, but this value can also increase depending on the pressure indicator. Typically, graphite is melted under pressure of 105 – 130 Bar.
MORPHOLOGY
Well-formed crystals are rare. The crystals are lamellar, scaly, curved, and usually have an imperfect lamellar shape. More often it is represented by leaves without crystallographic outlines and their aggregates. Forms continuous cryptocrystalline, foliated or rounded radial-radiating aggregates, less often – spherulitic aggregates of a concentric-zonal structure. Coarse-crystalline precipitates often exhibit triangular shading on the (0001) planes.
Story
Souvenir graphite block.
In terms of visual properties and description, graphite is similar to other minerals, so it is impossible to track the date of its first discovery. What is known is that the breed existed at least 6,000 years ago. This is evidenced by the pottery of Boyan Maritsa, a cultural settlement located on the territory of present-day Romania and Bulgaria. The pieces, dating back to approximately 4000 BC, are coated with graphite paints.
The name graphite was given by the German geologist Abraham Gottlob Werner for its property of leaving a color mark. In ancient Greek, “graphite” means “writing.”
In Russia, graphite was first discovered in 1826 in the Ural Mountains. Previously, the mineral was also called iron carbide and black lead.
ORIGIN
Formed at high temperatures in volcanic and igneous rocks, pegmatites and skarns. It is found in quartz veins with wolframite and other minerals in medium-temperature hydrothermal polymetallic deposits. Widely distributed in metamorphic rocks - crystalline schists, gneisses, marbles. Large deposits are formed as a result of pyrolysis of coal under the influence of traps on coal deposits (Tunguska basin). Accessory mineral of meteorites. Associated minerals: quartz, pyrite, garnets, spinel.
Graphite deposit
Graphite is in great demand in the industrial sector. About 600 million tons are considered reserves of the whole world, and 600 thousand are mined annually. The largest countries that mine this mineral are: Mexico, Russia, China, Czech Republic, South Korea, Canada, etc.
In addition to the countries mentioned above, there are other large deposits of graphite. For example, the island of Sri Lanka has been a major producer and supplier of this mineral since 1834. Mineral hotspots are found throughout the island, with graphite deposits concentrated in the central and southeastern parts. Two mined rocks are represented: Highland (granulites, quartz, charnockites) and Southwest (gneisses, calciphyres).
Scaly deposits of graphite in a huge proportion are located in Ukraine, in the Zavalevskoye deposit. This share is associated with the Archean formations of the Teterev-Bug series. The series is represented by sillimanite and garnet gneisses, quartz, crystalline limestone, etc. The mined minerals are of industrial importance and are also in demand.
APPLICATION
For the manufacture of melting crucibles, lining plates - the application is based on the high temperature resistance of graphite (in the absence of oxygen), on its chemical resistance to a number of molten metals. It is used in electrodes and heating elements due to its high electrical conductivity and chemical resistance to almost any aggressive aqueous solutions (much higher than that of noble metals). For the production of chemically active metals by electrolysis of molten compounds, solid lubricants, in combined liquid and paste lubricants, plastic filler.
It is a neutron moderator in nuclear reactors, a component of the composition for the manufacture of leads for black graphite pencils (mixed with kaolin). Used to produce synthetic diamonds, as a nanometer length standard for calibrating scanners of a scanning tunneling microscope and atomic force microscope, for the manufacture of contact brushes and current collectors for a variety of electrical machines, electric vehicles and overhead cranes with trolley power, powerful rheostats, as well as others devices that require reliable moving electrical contact for the manufacture of thermal protection for the nose of ballistic missile warheads and re-entry spacecraft.
Graphite – C
| Molecular weight | 12.01 g/mol |
| origin of name | from ancient Greek γράφω - to record, to write |
| IMA status | valid, first described before 1959 (before IMA) |
Boiling temperature
The need for heat treatment is driven by the fact that enterprises seek to modify the performance properties of the material in order to create more efficient products. Methods of bringing the mineral to a boil are less commonly used, but they can also improve certain properties of the structure. The question of what is the melting point and boiling point of graphite often involves indicating the same range - from 3800 to 4200 ° C. The lower threshold determines the melting state, and the upper threshold determines the boiling state of the material. Again, depending on the characteristics of graphite and its variety, the conditions of thermal action in terms of obtaining the desired state of the mineral - boiling or melting - may converge.
PHYSICAL PROPERTIES
| Mineral color | iron black fading to steel gray |
| Stroke color | black fading to steel gray |
| Transparency | opaque |
| Shine | semi-metallic |
| Cleavage | very perfect in {0001} |
| Hardness (Mohs scale) | 1-2 |
| Kink | mica-like |
| Strength | flexible |
| Density (measured) | 2.09 – 2.23 g/cm3 |
| Radioactivity (GRapi) | 0 |
Receiving technologies
Almost all graphite products undergo processing operations before final use. The method of production also determines the type of graphite material. As a rule, the difference in methods is determined by the temperature effect. Thus, by heating a mixture of pitch and coke, Acheson graphite is obtained. The melting and boiling points in this case will be 2800 and 4200 °C, respectively. The thermomechanical method of processing the coke mixture involves exposure to the same heating rates - the only difference is the use of carbide-forming components. The pyrolysis method is characterized by low temperature treatment rates. In this case, natural graphite is modified from gaseous hydrocarbons in a vacuum at 1500 °C. At the same time, cooling methods for processing base mixtures to produce graphite are also common. These technologies include blast furnace, during which the cast iron masses are slowly cooled.
Varieties
There are several types of natural graphite:
- crucible;
- crystal foundry;
- battery;
- for the production of pencils (fine, gray);
- elemental (for galvanic cells);
- electric coal
Natural graphite is also divided according to its form of occurrence in the earth’s crust:
- shungite (no longer coal, but not yet graphite);
- graphite (an amorphous type of mineral);
- graphite mica (another name for graphite).
Therapeutic effect
In folk medicine, graphite is in demand due to its healing properties. It is used for:
- treatment of skin diseases - psoriasis, eczema, dermatitis, lichen;
- elimination of psycho-emotional disorders - neurasthenia, depression, unreasonable fear;
- treatment of infertility in women and disorders of the reproductive system of the body;
- treatment of diseases of the ear, eyes, respiratory organs;
- normalization of the digestive system;
- improving metabolism;
- maintaining the thyroid gland.
The mineral is actively used in homeopathy under the name Graphites.
Where is it used?
The scope of application of graphite is very extensive. The mineral is used as a raw material for the manufacture of:
- refractory bricks and ceramics, plastic products;
- nuclear reactor rods used at nuclear power plants and other installations;
- heat-resistant containers;
- anti-corrosion coatings (paints, enamels);
- bearings, electrical appliances;
- pencils;
- lubricants for steel production;
- artificial diamonds.
Graphite is also added to medicines, alcohol, sugar, and paraffin.
Graphite by zodiac
Modern astrologers claim that graphite is mainly suitable only for Aries. This is due to the fact that the owners of this zodiac sign are characterized by the following traits: tough character, impulsiveness, hot temper and aggressiveness. Aries often become obsessed with themselves, they do not know how to compromise and always defend their opinion, even if they are wrong. In society, Aries may find it difficult to find a common language with people because of their eccentricity.
A talisman with graphite helps Aries become softer in character and more understanding. The stone takes away the accumulated evil energy and transforms it into positive energy.
In addition, graphite is suitable for Scorpios, since they are quite vindictive and vindictive, like to keep everything under control and are usually associated with the pressure of aggression and ambition. Graphite will give these zodiac signs such qualities as easygoingness and the ability to compromise.
The influence of graphite on other zodiac signs is insignificant.
Storage and care
Graphite has low hardness and extremely high brittleness. The main condition for storing this mineral is the exclusion of even minor mechanical influence. It should absolutely not be dropped or squeezed. It is recommended to purchase collector's items in a special box.
Graphite is resistant to chemicals, so if it gets dirty it can be washed with detergents. After rinsing, the stone should be left in the open air until completely dry.