Hello dear readers. As you know, diamond, despite its fascinating appearance, is a simple substance. In this article you will learn what the diamond formula is and what properties it provides.
The stone behaves quite non-standardly in many situations; many experiments and determination of certain values are difficult because of this. However, the properties of the stone are so high that various studies are still being carried out, hypotheses are put forward, and attempts continue to create analogues and even substances that surpass diamond in their properties.
Structure
The unit cell of the diamond crystal lattice.
The unit cell is crystalline. The A. lattice has the form of a cube, in which carbon atoms are located at the vertices, at the centers of its faces, as well as at the centers of 4 non-adjacent octants of the cube. Each carbon atom is bonded to the four nearest (symmetrically located along the vertices of the tetrahedron) covalent chemical. connection and is located at a distance of 0.154 nm from each of them. An ideal crystal of A. can be imagined as one giant molecule. Real crystals of aluminum always contain impurities (Si, Al, Ca, Mg, Na, Ba, Mn, Fe, Cr, Ti, B, H, N, and other elements) and lattice defects. Of the impurities, the greatest influence on the physical. properties of A. are provided by nitrogen, which is isomorphically included in the structure and forms (independently or in combination with structural defects) centers responsible for color, luminescence, absorption (in the ultraviolet, visible, infrared and microwave regions), the nature of the scattering of x-rays, etc.
Allotropic modifications
Some other chemical elements have a similar structure to diamond, but a slightly different molecular crystal lattice. The difference is in the arrangement of the atoms.
In diamond, the carbon atoms are located close to each other. And for other elements with a higher atomic mass, the distance between the atoms is greater, which reduces their strength.
Known allotropic modifications are:
- Lonsdaleites are not well studied, are mined from meteorites or created artificially, and have a hexagonal crystal lattice.
- Graphite - has a similar structure, but differs in pi bonds and the presence of free electrons (hexagonal crystal lattice).
- Coal is used as a raw material for heat generation.
- Carbin are small black crystals in powder form, artificially created.
- Fullerenes - a crystal lattice looks like a ball, assembled from octagons, artificially created.
- Carbon nanotubes are used as a framework for nanoproducts.
Allotropic modifications are capable of transformation: under the influence of a temperature of 1800 degrees they are converted into graphite.
Additionally, watch the video about allotropic forms of carbon - diamond and graphite:
Morphology
The “50 Years of October” diamond is octahedral in shape, weighing 121.66 carats. Tube "Mir". Yakutia (Russia).
A. crystallizes into cubic systems. The most important crystallographic A. forms: flat-sided (arising during crystal growth) - octahedron, rhombic dodecahedron, cube and various combinations thereof; curved (formed when crystals dissolve) - dodecahedroids, octahedroids and cuboids; There are also more complex combined forms, twins of intergrowth (according to the spinel law) and germination. The edges of crystals are often covered with figures of growth and dissolution - protrusions, depressions and steps. Among polycrystalline Aggregates include ballas (spherical spherulites with a radial structure), intergrowths, carbonado (hidden and microcrystalline aggregates of irregular shape, dense or slag-like), carbonado with lonsdaleite (hexagonal modification of carbon) and board (irregularly shaped fine- and coarse-grained polycrystalline formations). A.'s size ranges from microscopic. grains to very large crystals weighing hundreds and thousands of carats. The weight of mined diamonds is usually 0.1–1.0 ct (1 ct = 0.2 g); large crystals of St. 100 carats are very rare; As a rule, such stones are given their own names (Table 1). From the largest one, “Cullinan”, 105 diamonds were made, including “Star of Africa” (“Cullinan I”) of 530.2 carats and “Cullinan II” of 317.4 carats, which are inserted into the imp. crown and scepter of Great Britain. The largest grew. A. (342.5 ct) was found in Yakutia (Mir pipe).
Table 1. The world's largest diamonds
| Name | Weight, car | Place of discovery - country, mine | Year of discovery | Number of diamonds obtained from a diamond | Weight of the largest diamonds, carats |
| "Cullinan" | 3106,0 | South Africa, Premier | 1905 | 105 | 530,2; 317,4 |
| Untitled | 1640 (broken) | South Africa, Premier | 1919 | … | … |
| Untitled | 1195,5 | South Africa, Premier | 1924 | … | … |
| "Excelsior" ("Excelsior") | 971,50 | South Africa, Jachersfontein | 1893 | 21 | 69,8; 47,15 |
| "Star of Sierra Leone" | 968,90 | Sierra Leone, Yengema | 1972 | 17 | 153,96 |
| "Great Mogul" | 778,00 | India, Golconda | 1304? | … | 280 |
| "Victory Diamond" | 770,00 | Sierra Leone, Koidu | 1945 | 30 | 31,35 |
| "Nameless Brown" | 775,5 | South Africa, Premier | 1986 | 1, "Golden Jubilee", the world's largest | 545,67 |
| "President Vargas" | 726,60 | Brazil, Diamantina | 1938 | 29 | 48,26 |
| "Jonker" ("Jonker") | 726,00 | South Africa, Transvaal | 1934 | 12 | 125,65 |
| "Anniversary" | 650,80 | South Africa | 1895 | 2 | 245,35 |
| "Dutoitspan" | 616,00 | South Africa, Duthoitspan | 1974 | Not cut | |
| "Baumgold" | 609,25 | South Africa | 1922 | 14 | 50,00 |
| "Lesotho Brown" | 601,25 | Lesotho, Letseng la Terae | 1967 | 18 | 71,73 |
A little about prey and color
Several versions of the formation of diamonds are known:
- The result of increasing the temperature of silicates, which are compounds of oxygen and silicon. Crystals are hidden in the crust of the Earth's mantle, and powerful deep-seated explosions push them to the surface.
- Formation under the simultaneous influence of high temperatures and pressure caused by falling meteorites.
The mineral is mined from:
- Diamond mines by washing river and sea sand. This is how small pebbles are found, the carbon composition of which is designated by the letter C.
- Diamond quarries are places where rock deposits are located near the surface, which allows for open-pit mining.
- Underground mines, such as kimberlite pipes , first discovered in the 19th century. Ore is mined by drilling and laying mines.
- Diamond mines requiring a combination of work methods.
An untreated gemstone is unsightly in appearance and has a rough surface without shine. Crystals can be either microscopic or very large. The weight of most diamonds rarely exceeds 15 carats. The exception is nuggets weighing hundreds of carats. How much does 1 carat of diamond weigh →
Jewelers are interested in no more than 25% of all mined stones. The rest are destined to become part of industrial plants and tools. The smallest gems are turned into diamond powder.
The color range of diamonds is varied: watery-colorless, gray, blue, blue, yellowish, red, pink, green, black.
Many specimens are unevenly colored:
- zonally, for example, only in the upper part;
- spots.
The quality of a mineral is determined not only by color or size, but also by the presence/absence of inclusions and defects. The variety of colors determines the chemical composition of the diamond and the natural conditions under which its formation occurs. The incredible diamond hardness also depends on this factor.
Physical properties
There are A. colorless, yellowish, brown, green, blue, indigo, pink (of different shades and color intensity), milky white, gray (to black). When irradiated with charged particles, a colorless crystal becomes green or blue. Density of decomp. varieties ranges from 3470 to 3560 kg/m3 (for carbonado from 3010 to 3470 kg/m3). Hardness on the Mohs scale is 10. The anisotropy of aluminum determines many of its properties, for example. decomposition hardness according to different crystallographic directions (the hardest is the octahedral face), which allows it to be processed with a diamond tool. A. is fragile (in the presence of defects or inclusions), has perfect cleavage along the faces of the octahedron, bright brilliance due to the high refractive index of 2.417 (for a wavelength of 0.5893 microns), and a strongly pronounced dispersion effect, providing an iridescent play of light on the facets of the diamond . The degree of transparency depends on the number of inclusions - solid (graphite, olivine, pyroxene, garnets, chrome spinels, coesite and other minerals) and gas-liquid. As a rule, crystals exhibit anomalous birefringence due to stresses arising from structural defects and inclusions. Most crystals exhibit luminescence (in the green and blue parts of the spectrum) under the influence of ultraviolet, x-ray and gamma radiation, as well as phosphorescence. The clean surface of crystals is highly hydrophobic; in nature, thin films are formed on the surface, increasing wettability. A. is a dielectric (electrical resistivity varies in the range of 1–1010 Ohm cm), diamagnetic, and has high thermal conductivity (for some crystals at room temperature it is 4 times higher than the thermal conductivity of copper). The polymorphic transition of aluminum into graphite at atmospheric pressure occurs at a temperature of 1885 ± 5 ° C throughout the entire volume of the crystal. In air it burns at a temperature of St. 850 °C.
Diamond, characteristics, description, crystal lattice, chemical composition:
Diamond (from ancient Greek ἀδάμας “indestructible”, through Arabic ألماس ['almās] and Turkish elmas) is a mineral, a cubic allotropic form of carbon. The chemical formula of diamond is C.
Diamond
is a natural mineral consisting of carbon and crystallizing in the cubic system.
Along with graphite and diamond, there are many more allotropic forms of carbon. For example, graphene, fullerene, carbon nanotubes, etc. The properties of these substances are completely different from each other.
Diamond is the hardest natural material on Earth.
Diamond is a rare, but at the same time quite widespread mineral. To date, diamonds have been found on all continents of the Earth, including Antarctica.
Diamond is a solid allotropic form of carbon whose atoms have a face-centered cubic crystal lattice. Moreover, each carbon atom in the diamond structure is located in the center of a tetrahedron, the vertices of which are the four nearest atoms.
Thus, in a diamond, each carbon atom is bonded to four other atoms. In diamond, the bonds are formed by sp3 hybrid orbitals. This connection is the strongest. It is the strong bond of carbon atoms and the absence of a free electron that explains the high hardness of diamond . Of all known substances, diamond also has the largest number of atoms per unit volume, making it both the hardest and least compressible.
Conversely, in graphite, another allotrope of carbon, each carbon atom is bonded to three similar atoms and has one free electron. In graphite, interatomic bonds are formed by sp2 hybrid orbitals. Bonds between carbon atoms in graphite are formed in the same plane. The bonds between graphite planes are weak. This determines the high softness of graphite and the ability of graphite layers to easily separate (peel off) from each other.
Under normal conditions (i.e. room temperature and normal pressure), as well as high pressures, diamond can exist indefinitely. At room temperature and pressure, another solid form of carbon known as graphite is also a chemically stable form, but diamond almost never transforms into it. And only in a vacuum or in an inert gas at elevated temperatures - at 2000 oC, diamond gradually turns into graphite.
Diamonds come in completely different colors and shades: from steel gray, white to brown and black. Colorless and transparent stones are rare. This is because natural diamond may contain small amounts of defects and impurities (about one per million carbon atoms). Small amounts of defects or impurities color a diamond blue (boron impurities), yellow (nitrogen impurities), brown (lattice defects), green (radiation effects), purple, pink, orange, red or gray. At the same time, a chemically pure and structurally perfect diamond is transparent and has no tint or color.
Diamond also has relatively high optical dispersion (the ability to scatter light of different colors).
Diamond's hardness and high optical dispersion contribute to its use as a gemstone. Unlike many other gemstones, it is well suited for daily wear due to its scratch resistance. A diamond can only be scratched by
A cut diamond is called a diamond.
Diamond is made of pure carbon. In small quantities it contains various impurities of other chemical elements (boron, nitrogen, aluminum, silicon, calcium, magnesium, etc.).
Prevalence and origin
A. is found in kimberlites, lamproites, and “related” igneous rocks. rocks (some types of lamprophyres, picrites, peridotites), as well as in decomposition. placers. A., sharply different from the above types (carbonado, carbonado with lonsdaleite), are sometimes found in impactites that fill astroblemes, in metamorphic. rocks, as well as in meteorites. There is no consensus on the origin of A. Origin of A. kimberlite-lamproitic type b. h. Scientists connect with the upper mantle of the Earth, with the depths of St. 150 km, where it crystallizes into deep igneous rocks. ultrabasic (peridotites) and basic (eclogites) rocks and is located there at extremely high pressures (40–65, up to 1.05 107 kPa) and temperatures of approx. 1150–1350 °C. Most researchers recognize that kimberlite or lamproite magma (filling diatremes), previously considered to be many others. researchers study the environment of A. formation, plays the role of a transporter in this process. Depending on its properties (temperature, the rate of rise of the magmatic column, the content of various chemical elements, especially alkaline ones), it can both preserve the material quite well and be extremely aggressive towards it - up to its complete dissolution and/or conversion to graphite. Genesis of A. in metamorphic. breeds b. Many researchers explain their formation at low temperatures and pressures in the upper horizons of the earth's crust simultaneously with the formation of host rocks. According to an alternative point of view, these A. are initially associated with the mantle diamond-bearing ultrabasic rock, which penetrated into the upper horizons of the crust, then transformed there (almost completely changing its chemical and mineral composition) as a result of the influence of metasomatics. processes. Formation of polycrystalline. A. (carbonado, carbonado with lonsdaleite), contained in astrobleme impactites, is explained by the recrystallization of graphite of ancient metamorphic rocks. graphite-containing rocks as a result of the so-called. shock metamorphism of impact (intrusion of a large meteorite) or explosive nature (endogenous explosive structure).
Mechanical, optical, chemical and other properties of diamond:
– Diamond is a solid form of pure carbon. Hardness on the Mohs scale 10,
– the hardness of a diamond depends on its purity, the absence of crystal lattice defects and orientation. Hardness is higher for flawless, clear crystals oriented along the longest diagonal of the cubic diamond lattice. Therefore, diamonds can only be scratched and processed by other diamonds,
– Of all known substances, diamond has the largest number of atoms per unit volume, so it is both the hardest and least compressible. Diamond has the lowest compression ratio,
– has a high density of 3.47-3.55 g/cm³,
– is brittle and breaks easily,
– conchoidal fracture,
– has a high refractive index and relatively high optical dispersion (the ability to scatter light of different colors). These properties make the edges applied during diamond processing shine, playing in the light,
– has the highest thermal conductivity among all solids 900-2300 W/(m K). Because of this, the diamond feels cold to the touch.
– diamond has a very low coefficient of friction on metal,
– has the highest modulus of elasticity,
– in air, diamond burns at 850-1000 °C, and in a stream of pure oxygen it burns with a faint blue flame at 720-800 °C, completely turning into carbon dioxide,
– under the influence of sunlight, as well as under the influence of cathode, ultraviolet and x-rays, diamonds begin to luminesce - glow in different colors. It is this specific property of diamond that allows it to be identified in the rock,
– the surface of diamond is hydrophobic and lipophilic , i.e. diamond is not wetted by water, but is well wetted by oil and fat. This property is used to distinguish a diamond from a fake. The fat on the fake does not completely wet the surface, but collects in small droplets. In addition, a diamond smeared with grease sticks to the glass, but a fake does not,
– diamonds are chemically stable. At room temperature they do not react with acids and alkalis. The surface of a diamond can only oxidize at air temperatures above 850 °C. Diamond also reacts with fluorine gas at temperatures above 700 °C,
– the refraction of diamond is such that if you place a colorless crystal on a page with printed text, you will not be able to read what is written. This characteristic of a diamond allows you to distinguish a fake from an original. Also, if you look through a diamond at the sun, you will only see a dim point,
– under the influence of radioactive radiation, the diamond changes color to a rich green color.
Place of Birth
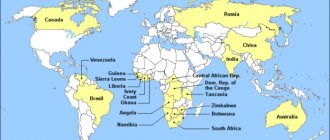
Manifestations of A. are known in 43 countries, industrial. deposits have been identified in 26. The total reserves of A., established in 25 countries (excluding Russia), amount to 991 million carats (Table 2), including in Africa (54.5%), Australia (9.6% ), South and Sev. America (34.8), Asia (0.6%). The largest diamond reserves (foreign) are concentrated in the South African, Central African, Canadian, West African, Western Australian, and South American diamond-bearing provinces. OK. 85% of the known reserves of aluminum are concentrated in primary deposits, the rest in placers. Primary deposits have been identified: in igneous. rocks – kimberlites (in pipes, dikes, sills; e.g. Botswana, Russia, South Africa, Canada, Angola, Sierra Leone); lamproites (pipes, dikes; Australia, India), in their ancient metamorphosed varieties - “phyllites” and talc schists (Brazil, Ghana, Guyana); impactites (Popigai astrobleme, Yakutia, Russia); metamorphic rocks - eclogites, carbonate metasomatites, gneisses (Kazakhstan). Prom. A. deposits in bedrock are so far known only in kimberlites (almost all) and olivine lamproites (the largest deposit in the world in terms of reserves is Argyle, Australia). The only metamorphogenic deposit of A. is Kumdykolskoe (Kazakhstan) and shock-metamorphic. deposits in the Popigai astrobleme (the largest - Skalnoye and Udarnoye - significantly exceed in reserves the known deposits of traditional genetic types) do not yet represent industrial. interest, because they contain small and difficult to extract A. specific. morphology and structure. St. There are 6,500 kimberlite bodies, but only a few of them are industrially diamond-bearing. dozens. The A content in primary deposits varies widely from 0.1–7 carats/t (rarely more). In most primary deposits, the most valuable jewelry varieties of diamonds account for 5–15% by number of crystals, 40–70% by weight, and 97.5–98% by value. In terms of A. content, the largest is the unique Yakut. “International” pipe (up to 17 carats/t), at the cost of 1 ton of ore – Yakut. pipe “Name of the XXIII Party Congress” - up to $700/t, based on the total cost of extracted diamonds - Jwaneng deposit (Botswana). The largest industrial Indigenous deposits of A. are located in Botswana, Russia (Yakutia, Arkhangelsk region), South Africa, Australia, Canada, Angola, Democratic. Republic of the Congo.
Table 2. The largest resources and reserves of natural diamonds, including jewelry, by country and the total world (without Russia; estimate by the beginning of 2004), million carats
| A country | Natural Diamond Resources | Total reserves of natural diamonds | Jewelry diamond reserves |
| Asia | |||
| India | 10 | 1 | 1 |
| China | 35 | 5 | 2 |
| Angola | 730 | 92 | 80 |
| Botswana | 950 | 185 | 140 |
| Ghana | 15 | 5 | 2 |
| Guinea | 30 | 1 | 0,8 |
| Africa | |||
| Democracy. Republic of the Congo | 350 | 65 | 6 |
| Zimbabwe | 50 | 35 | 20 |
| Lesotho | 30 | 8 | 4 |
| Namibia | 930 | 10 | 9,5 |
| Sierra Leone | 50 | 12 | 8 |
| CAR | 22 | 2 | 1,5 |
| South Africa | 450 | 125 | 55 |
| America | |||
| Brazil | 40 | 3 | 2,5 |
| Venezuela | 90 | 67 | 30 |
| Canada | 800 | 275 | 64 |
| Australia | |||
| Australia | 500 | 95 | 28 |
| Other countries | 118 | 5 | 4 |
| Total in the world | 5200 | 991 | 458,3 |
Placer deposits are associated with alluvial formations of channels, floodplains, terraces of modern and ancient river valleys, as well as marine terrestrial and underwater terraces and paleovalleys of rivers. Prom. Eluvial, deluvial and karst placers are also important. The A. content in placers is 0.001–5 carats/m3 (sometimes reaches tens of carats per m3). The quality (as well as size and, accordingly, price) of diamonds in placers is significantly higher than in primary deposits: in some placers in South Africa it exceeds $1,200/car, in Yakutia – approx. 60–80, in the Urals – up to $350/car. The largest placer deposits have been identified in Democracy. Republic of Congo, Namibia, South Africa, Angola, Ghana, Brazil, etc. Main. reserves of jewelry raw materials are concentrated in Botswana, Russia, Angola, Canada, South Africa, Venezuela, Australia; technical A. - in Canada, Russia, South Africa, Australia, Democratic. Republic of Congo, Botswana, Venezuela.
Russia occupies a leading place in the world both in terms of explored reserves of aluminum and in predicted resources. Explored reserves are concentrated in primary deposits (94.6%, kimberlites) and placers (5.4%). The first gold mines in the country were discovered in the Ural diamond-bearing province in 1829 in gold-bearing alluvial placers on Sr. Urals (in the Koiva River basin). Placers of the Urals are characterized by a high yield of jewelry stones (average weight 0.15–1.0 ct), but their reserves are limited (approx. 0.1% of all-Russian ones); no indigenous sources have yet been discovered. Basic Some of the deposits are located in the Yakut diamond-bearing province and the Arkhangelsk diamond-bearing region. In the 1970s The high diamond content of the impactites of the Popigai astrobleme was revealed, which is associated with the products of melting of the “target” rocks (tagamites) or their explosive destruction (suvites). In most cases, diamond-bearing bodies of tagamites and suvites have a sheet-like shape, subhorizontal occurrence, and are characterized by a stable diamond content along strike and depth. A. are occasionally found in the Kara, Puchezh-Katun and other astroblemes of Russia.
APPLICATION
Good crystals are cut and used in jewelry. About 15% of mined diamonds are considered jewelry, another 45% are considered near-jewelry, that is, inferior to jewelry in size, color or clarity. Currently, global diamond production is about 130 million carats per year. Diamond
(from the French brillant - brilliant), is a diamond that has been given a special shape through mechanical processing (cutting), a brilliant cut, which maximizes the optical properties of the stone such as brilliance and color dispersion.
Very small diamonds and fragments, unsuitable for cutting, are used as an abrasive for the manufacture of diamond tools necessary for processing hard materials and cutting the diamonds themselves. The cryptocrystalline variety of diamond, black or dark gray in color, forming dense or porous aggregates, is called Carbonado
, has a higher abrasion resistance than diamond crystals and is therefore especially valued in industry.
Small crystals are also grown artificially in large quantities. Synthetic diamonds are obtained from various carbon-containing substances, mainly from graphite, in special. apparatuses at 1200-1600°C and pressures of 4.5-8.0 GPa in the presence of Fe, Co, Cr, Mn or their alloys. They are suitable for technical use only.
Diamond – C
| Molecular weight | 12.01 g/mol |
| origin of name | From Greek, adamas, meaning “invincible” or “solid.” |
| IMA status | valid, first described before 1959 (before IMA) |
Use of natural diamonds
On the world market, there are 3 types of diamonds: jewelry (transparent single crystals of perfect shape, weighing at least 0.05 ct, with a small number of inclusions and other defects), subjewelry (with a slightly larger number of defects) and technical. Jewelry and sub-jewelry diamonds cut with a special brilliant cut are called diamonds; the rest are used in technical applications. purposes. A certain amount of jewelry-quality aluminum is also used in technical applications. purposes, mainly in the field of electronics and other high-tech industries (with the ratio of the price of raw materials to the price of a device manufactured on its basis no more than 1:100), but their share is small due to their high cost.
Table of molar masses of chemical elements:
| Atomic number | Chemical element | Symbol | Molar mass |
| 1 | Hydrogen | H | 1.00784-1.00811 amu (g/mol) |
| 2 | Helium | He | 4.002602(2) amu (g/mol) |
| 3 | Lithium | Li | 6.938-6.997 amu (g/mol) |
| 4 | Beryllium | Be | 9.012182(3) amu (g/mol) |
| 5 | Bor | B | 10.806-10.821 amu (g/mol) |
| 6 | Carbon | C | 12.0096-12.0116 amu (g/mol) |
| 7 | Nitrogen | N | 14.00643-14.00728 amu (g/mol) |
| 8 | Oxygen | O | 15.99903-15.99977 amu (g/mol) |
| 9 | Fluorine | F | 18.998403163(6) a.m.u. (g/mol) |
| 10 | Neon | Ne | 20.1797(6) amu (g/mol) |
| 11 | Sodium | Na | 22.98976928(2) amu (g/mol) |
| 12 | Magnesium | Mg | 24.304-24.307 amu (g/mol) |
| 13 | Aluminum | Al | 26.9815386(8) amu (g/mol) |
| 14 | Silicon | Si | 28.084-28.086 amu (g/mol) |
| 15 | Phosphorus | P | 30.973762(2) amu (g/mol) |
| 16 | Sulfur | S | 32.059-32.076 amu (g/mol) |
| 17 | Chlorine | Cl | 35.446-35.457 amu (g/mol) |
| 18 | Argon | Ar | 39.948(1) amu (g/mol) |
| 19 | Potassium | K | 39.0983(1) amu (g/mol) |
| 20 | Calcium | Ca | 40.078(4) amu (g/mol) |
| 21 | Scandium | Sc | 44.955912(6) amu (g/mol) |
| 22 | Titanium | Ti | 47.867(1) amu (g/mol) |
| 23 | Vanadium | V | 50.9415(1) amu (g/mol) |
| 24 | Chromium | Cr | 51.9961(6) amu (g/mol) |
| 25 | Manganese | Mn | 54.938045(5) amu (g/mol) |
| 26 | Iron | Fe | 55.845(2) amu (g/mol) |
| 27 | Cobalt | Co | 58.933194(4) amu (g/mol) |
| 28 | Nickel | Ni | 58.6934(4) amu (g/mol) |
| 29 | Copper | Cu | 63.546(3) amu (g/mol) |
| 30 | Zinc | Zn | 65.38(2) amu (g/mol) |
| 31 | Gallium | Ga | 69.723(1) amu (g/mol) |
| 32 | Germanium | Ge | 72.630(8) amu (g/mol) |
| 33 | Arsenic | As | 74.92160(2) amu (g/mol) |
| 34 | Selenium | Se | 78.96(3) amu (g/mol) |
| 35 | Bromine | Br | 79.901-79.907 amu (g/mol) |
| 36 | Krypton | Kr | 83.798(2) amu (g/mol) |
| 37 | Rubidium | Rb | 85.4678(3) amu (g/mol) |
| 38 | Strontium | Sr | 87.62(1) amu (g/mol) |
| 39 | Yttrium | Y | 88.90585(2) amu (g/mol) |
| 40 | Zirconium | Zr | 91.224(2) amu (g/mol) |
| 41 | Niobium | Nb | 92.90638(2) amu (g/mol) |
| 42 | Molybdenum | Mo | 95.96(2) amu (g/mol) |
| 43 | Technetium | Tc | 97.9072 amu (g/mol) |
| 44 | Ruthenium | Ru | 101.07(2) amu (g/mol) |
| 45 | Rhodium | Rh | 102.90550(2) amu (g/mol) |
| 46 | Palladium | Pd | 106.42(1) amu (g/mol) |
| 47 | Silver | Ag | 107.8682(2) amu (g/mol) |
| 48 | Cadmium | Cd | 112.411(8) amu (g/mol) |
| 49 | Indium | In | 114.818(1) amu (g/mol) |
| 50 | Tin | Sn | 118.710(7) amu (g/mol) |
| 51 | Antimony | Sb | 121.760(1) amu (g/mol) |
| 52 | Tellurium | Te | 127.60(3) amu (g/mol) |
| 53 | Iodine | I | 126.90447(3) amu (g/mol) |
| 54 | Xenon | Xe | 131.293(6) amu (g/mol) |
| 55 | Cesium | Cs | 132.9054519(2) amu (g/mol) |
| 56 | Barium | Ba | 137.327(7) amu (g/mol) |
| 57 | Lanthanum | La | 138.90547(7) amu (g/mol) |
| 58 | Cerium | Ce | 140.116(1) amu (g/mol) |
| 59 | Praseodymium | Pr | 140.90765(2) amu (g/mol) |
| 60 | Neodymium | Nd | 144.242(3) amu (g/mol) |
| 61 | Promethium | Pm | 144.9127 amu (g/mol) |
| 62 | Samarium | Sm | 150.36(2) amu (g/mol) |
| 63 | Europium | Eu | 151.964(1) amu (g/mol) |
| 64 | Gadolinium | Gd | 157.25(3) amu (g/mol) |
| 65 | Terbium | Tb | 158.92535(2) amu (g/mol) |
| 66 | Dysprosium | Dy | 162,500(1) amu (g/mol) |
| 67 | Holmium | Ho | 164.93032(2) amu (g/mol) |
| 68 | Erbium | Er | 167.259(3) amu (g/mol) |
| 69 | Thulium | Tm | 168.93421(2) amu (g/mol) |
| 70 | Ytterbium | Yb | 173.045(10) amu (g/mol) |
| 71 | Lutetium | Lu | 174.9668(1) amu (g/mol) |
| 72 | Hafnium | Hf | 178.49(2) amu (g/mol) |
| 73 | Tantalum | Ta | 180.94788(2) amu (g/mol) |
| 74 | Tungsten | W | 183.84(1) amu (g/mol) |
| 75 | Rhenium | Re | 186.207(1) amu (g/mol) |
| 76 | Osmium | Os | 190.23(3) amu (g/mol) |
| 77 | Iridium | Ir | 192.217(3) amu (g/mol) |
| 78 | Platinum | Pt | 195.084(9) amu (g/mol) |
| 79 | Gold | Au | 196.966569(4) amu (g/mol) |
| 80 | Mercury | Hg | 200.592(3) amu (g/mol) |
| 81 | Thallium | Tl | 204.382-204.385 amu (g/mol) |
| 82 | Lead | Pb | 207.2(1) amu (g/mol) |
| 83 | Bismuth | Bi | 208.98040(1) amu (g/mol) |
| 84 | Polonium | Po | 208.9824 amu (g/mol) |
| 85 | Astatine | At | 209.9871 amu (g/mol) |
| 86 | Radon | Rn | 222.0176 amu (g/mol) |
| 87 | France | Fr | 223.0197 amu (g/mol) |
| 88 | Radium | Ra | 226.0254 amu (g/mol) |
| 89 | Actinium | Ac | 227.0278 amu (g/mol) |
| 90 | Thorium | Th | 232.03806(2) amu (g/mol) |
| 91 | Protactinium | Pa | 231.03588(2) amu (g/mol) |
| 92 | Uranus | U | 238.02891(3) amu (g/mol) |
| 93 | Neptunium | Np | 237.0482 amu (g/mol) |
| 94 | Plutonium | Pu | 244.0642 amu (g/mol) |
| 95 | Americium | Am | 243.061375 amu (g/mol) |
| 96 | Curium | Cm | 247.0703 amu (g/mol) |
| 97 | Berkelium | Bk | 247.0703 amu (g/mol) |
| 98 | Californium | Cf | 251.0796 amu (g/mol) |
| 99 | Einsteinium | Es | 252.083 amu (g/mol) |
| 100 | Fermium | Fm | 257.0951 amu (g/mol) |
| 101 | Mendelevium | MD | 258.1 amu (g/mol) |
| 102 | Nobelium | No | 259.1009 amu (g/mol) |
| 103 | Lawrence | Lr | 266 amu (g/mol) |
| 104 | Rutherfordium (Kurchatovium) | Rf | 267 amu (g/mol) |
| 105 | Dubnium (Nilsborium) | Db | 268 amu (g/mol) |
| 106 | Seaborgium | Sg | 269 amu (g/mol) |
| 107 | Borius | Bh | 270 amu (g/mol) |
| 108 | Hassiy | Hs | 269 a. e.m. (g/mol) |
| 109 | Meitnerium | Mt | 278 a. e.m. (g/mol) |
| 110 | Darmstadt | Ds | 281 a. e.m. (g/mol) |
Demand factor 4,720
Synthetic diamonds
Synthetic diamonds are obtained from graphite and carbon-containing substances starting from sulfur. 1950s There are three main ones. synthesis method A. At high pressures (above 4 GPa) and high temperatures (above 1100 ° C), diamond powders and large (weighing more than 1 carat) mono- and polycrystals are obtained. At low pressure chemical By deposition of hydrocarbons (mainly CH4) from the gas phase onto a substrate, mono- and polycrystalline materials are obtained. diamond films with a diameter of up to 100 mm and a thickness of up to 1 mm. By exploding carbon-containing substances (dynamic synthesis method) at ultra-high pressures and temperatures, ultrafine diamond powders are obtained - nanodiamonds with a particle size of 5–8 nm and a specific surface area of 250–350 cm2/g.
Synthetic A. Sec. arr. in cutting and grinding tools, as well as in semiconductor electronics and precision instrumentation; Large, high-quality single crystals are used to make jewelry.
Method of education
Diamond has been one of the most expensive precious stones for many centuries. Questions about genesis were controversial until recently. Back in the 50s of the last century, it was argued about their plant origin, and Geppert’s monograph on this was awarded the highest awards and recognition from the Dutch scientific community. This state of affairs did not last long, namely, the discovery of craters in Africa and meteorites with diamond inclusions changed the established opinion about the origin of this stone.
The main type of deposits are pipes formed by an explosion, they are also called diatremes. These diatremes are mainly composed of kimberlites - brecciated igneous rocks. Kimberlites also contain ancient deep rocks - xenoliths. There is an assumption that it is they who make up the upper mantle of the Earth.
Deep rocks with diamonds brought to the surface by an explosion, as well as mineral inclusions, indicate the formation of the latter under the influence of high pressure and temperature. These two indicators indicate a certain depth facies (110-135 km) at pressures of 4-4.3 GPa. If you translate it into the usual “a”, you get approximately 40 thousand atmospheres. Today it is believed that diamond in the pipes was formed at a depth of 100-200 km at temperatures of 1300-1700oC and higher pressures.
Despite the conditions of formation, diamond on the earth's surface is an unstable stable carbon modification. According to our ideas, it should have been transformed into graphite, but this did not happen.
History of mining in the world
The first country where diamond mining began was India. In the sacred Indian books - the Vedas - diamond is mentioned several thousand years BC. The diamond-bearing region extended over a large area of the mountainous part of India, called the Deccan, stretching from the Penner River in the state of Madras in a northern direction to the Son and Ken rivers flowing into the island. Ganges in the province of Pradesh. The largest Indian diamonds, Kokhinoor, Orlov and others, were found in the rich Golconda mines, located in the lower reaches of the Kistna River, near the city of Ellura.
Production in India
For a long time, the methods of diamond mining in India were shrouded in deep secrecy. The owners of the stones deliberately invested the diamond with mystery in order to raise its price. Therefore, in Indian literature, truth was so mixed up with fiction that it was impossible to separate them from each other. A.E. Fersman, in his book “Essays on the History of Stone,” cites one such legend found in Aristotle’s stories about precious stones. Diamonds in India and Ceylon were found in valleys so deep that the bottom was not visible. When Alexander the Great came across such a valley during his campaign in India, he wished to get diamonds. However, none of the people dared to go down into the abyss where there were poisonous snakes. On the advice of the sages accompanying him, Alexander ordered pieces of raw meat to be thrown to the bottom of the abyss. The birds of prey flying behind the army, descending for meat, picked up the diamonds that had stuck to it. Diamonds mined in this way were the size of lentils, sometimes as big as half a pea. This legend is found in Indian literary sources in different versions. Stories about mining diamonds from inaccessible abysses with the help of birds were widespread in ancient literature. They are available in Epiphanius of Cyprus, in the Armenian collection on stones, in the Russian ABC Book, in Marco Polo and others. These legends were wittily ridiculed at the beginning of our era by the outstanding Uzbek naturalist Biruni (973-1048). This is what he wrote in his book “Collected Information for the Knowledge of Jewels (Mineralogy)”: “Many fables are told about diamond mines and how diamonds are found. So, among the diamond’s nicknames is the name “Eagle Stone”; and it was given to him, as they say, because diamond seekers cover a nest with eagle chicks with glass, and the eagle, seeing him and not being able to penetrate the nest, flies away, brings the diamond and places it on the glass. When a lot of diamonds have been collected, the seekers take them and remove the glass, so that the eagle will think that he has achieved success in what he did; after a while they again put the glass on the nest and the eagle again brings diamonds... the story as a whole is stupidity, nonsense and fiction. Equally absurd is the statement that all the diamonds that exist now are those that Dhu-l-Qarnain mined from the valley (of diamonds). There were snakes there that people died from looking at. And so he ordered a mirror to be carried in front, behind which those who carried it were hiding. When the snakes saw themselves (in the mirror), they immediately died. But even before that, one snake saw another and did not die, and yet the body itself would be more capable of killing than its reflection in the mirror. If what they say concerns only people, then why should the snake die when it sees itself in the mirror? And finally, if people learned what Dhu-l-Qarnain came up with, then what prevents them from repeating it? business after that? There are also people who claim, when talking about diamonds, that they are in an abyss where there is no passage or descent for anyone, and that the people who hunt them cut the body of the animal into pieces and throw pieces of fresh meat there, which fall on the diamonds , and they stick to them. And there are eagles and vultures flying there, who know these places and are accustomed to such actions of people, have ceased to be afraid of them and have become tamed to them. They grab the meat and carry it to the edge of the gorge, where they begin to devour it, shaking off everything that has stuck to it... Then people come and pick up what might fall from the diamonds there. That is why it is called “Eagle Stone”. And there is no end to this nonsense." Biruni. Collection of information for knowledge of jewelry. The spread of all kinds of legends was facilitated by the owners of diamonds themselves, since investing the stone with mystery and fables about the difficulties of its extraction helped set high prices. Meanwhile, diamond mining was carried out in a fairly simple and accessible way. Biruni indicates that diamond sand was washed in the same way as gold-bearing sand; the sand was washed away from the conical tray, and the diamond settled below. In India, as a rule, only high-quality large stones were mined, which could be used as jewelry in their natural form after grinding the edges. Diamonds unsuitable for this purpose were thrown into dumps. There was already a caste classification here in ancient times. White crystals belonged to the highest caste of “brahmins”, with a reddish tint - to “kshatriyas”, greenish ones - to “voishya”, gray ones - to “sudras”. The “Brahmins” had the highest Value, the “Sudras” the lowest. This was the first attempt to classify by color. Until the 10th century AD, India was the world's only supplier of diamonds. In the 6th-10th centuries AD, Indian emigrants penetrated the island of Borneo (Kalimantan) and discovered rich diamond deposits here in the basin of the Landak, Sikoyam and Sarawak rivers, which flow into the river. Kapuas in the west of the island. At the end of the 17th century, minerals were discovered on the Tana-Lyaut Peninsula (in the basin of the Martapura River and its tributaries Riam-Kiva, Riam-Kanan and Banjo-Irang), near the city of Banjermaski (in the southeast of the island). The island of Borneo, together with India, were the main suppliers until the middle of the 18th century, and only they supplied the world market with diamonds.
Production in Brazil
In 1695, in Brazil, in the state of Minas Gerais, prospector Anthony Rodrigo Ardao discovered the first diamonds while panning for gold in Tejuco (now Diamantina). But then, out of ignorance, they were not given much importance, and the found crystals were used as stamps during the game. This went on for almost 30 years. In 1725, Bernardo da Francesco Labo was the first to announce the discovery of diamonds. Lisbon experts confirmed that the stones found are indeed diamonds. A diamond rush has begun in Brazil. Prospectors - individuals and groups of enterprising people rushed to search for and mine diamonds. So many of the latter were mined that already in 1727, i.e. two years after Labo’s application, the price of diamonds fell sharply. In order to maintain high prices on the world market, diamond traders resorted to all sorts of tricks. Dutch traders, for example, who controlled the supply of diamonds from India, declared that no diamonds had been discovered in Brazil at all and that supposedly the so-called “Brazilian” diamonds were nothing more than low-grade Goan diamonds brought to Brazil, from where they were exported to Europe disguised as Indian. In the 70s of the 18th century, diamonds were discovered in the states of Goiás and Mato Grosso. Their production has increased even more. If from 1730 to 1740 200,000 carats were mined, then from 1741 to 1771 there were already 1,666,569 carats. The decline in diamond prices was halted by the Portuguese government, which imposed high taxes and such onerous conditions that diamond mining in Brazil ceased. In 1772, diamond mining was declared a state monopoly. In 1822, Brazil freed itself from Portuguese rule and became an independent state. The country's government has again allowed private individuals to mine diamonds. In 1844, Brazil's diamond industry received a new boost with the discovery of diamonds in the state of Bahia. It was here that black diamond, carbonado, was first discovered. For a century and a half, Brazil was the main supplier of stones to the world market, but then its glory faded due to the discovery of the richest South African deposits.
Production in Australia
In 1851, gold and tin placers were discovered during panning in Australia. But only the placers of New South Wales, discovered in 1859-1867, turned out to be industrial, where in some years up to 4,000 carats were mined. The increase in production occurred until 1915, when 186,963 carats were obtained, after which their production fell sharply due to the depletion of placers; it now produces just over 200 carats a year.
How to distinguish an original from a fake
When talking about methods for determining the authenticity of diamonds, it is worth distinguishing between testing the authenticity of diamonds and rough diamonds. An inexperienced person may confuse a diamond with quartz, crystal, other transparent minerals, and even glass. However, the exceptional physical and chemical properties of diamond make it easy to identify a fake.
First of all, it is worth remembering hardness. This stone can scratch any surface, but only another diamond can leave marks on it. Also, no perspiration remains on a natural crystal if you breathe on it. A wet stone will have a mark like a pencil if you run aluminum over it. You can check it with an X-ray: natural stone under radiation has a rich green color. Or look through it at the text: through a natural diamond it will be impossible to make out it. It is also worth noting that the naturalness of the stone can be checked by the refraction of light: by holding the original to a light source, you can only see a luminous point in the center.
Production in Russia
The first statement about the possibility of finding diamonds in Russia belongs to the founder of Russian mining science M.V. Lomonosov, who back in 1763 wrote in his treatise “The First Foundations of Metallurgy, or Ore Mining”: “By many evidences, I conclude that in the northern terrestrial in the depths of the earth, nature reigns extensively and richly... Considering this and imagining the time when elephants and grass from the southern lands were growing in the north, we cannot doubt that diamonds, yachts and other expensive stones could have appeared, and they could be found, as recently silver and gold , which our ancestors did not know." Later, in 1823, the famous naturalist of the 19th century A. Humboldt noted the similarity of the geology of the placers of the Urals and Brazil, where diamonds are found in placer deposits together with gold and platinum. According to this scientist, diamonds in the Urals should have been discovered soon.
In 1828, at a reception at the Russian court, Humboldt declared that he would not return from his trip to the Urals without the “first Russian diamond.” On July 5, 1829, in the Urals, in the area of the Holy Cross gold placer, 14-year-old Pavel Popov found the first diamond crystal, which weighed half a carat. Three days later, a second crystal weighing 2/3 carats was found, and a few days later a third crystal weighing 1/g carat was found. In subsequent years, they were discovered in other places of the Urals: on the eastern slope (1831), on the river. Kushayke is a left tributary of the river. Saldy (1838); at the Uspensky mine in Verkhneuralsky district (1839); along the river Silver (1876). The next discovery of a diamond dates back to 1884 at a placer along the river. Crane - tributary of the river. Is, in 1891 in the placer of the river. M. Sap, near the village of Ayatsky. In 1892, they were found on gold-bearing placers in the Southern Urals. One diamond was found near the village of Kochkar, the other - at the Viktorovsky mine along the river. Kamenka. Two diamonds were found along the river in 1895. Polozhikhe, near the village of Koltyshi. There are references to the discovery of two diamonds along the river. Bobrovka in Nizhne Tagil region. During the period from 1829 to 1858, 131 crystals weighing a total of 59.5 carats were found at the Krestovozdvizhensky gold mines, where the first diamond was found. In total, 239 diamonds with a total weight of 79,242 carats were found in the Urals from 1829 to 1920. The largest stone found weighed about 3 carats. Almost all the crystals were found by chance by prospectors while panning gold-bearing sands. Very few special searches for diamonds were carried out. There is information about such searches only in Adolfovsky Log (Ural). The owners of gold mines and the management of state-owned factories tried to organize ski. Thus, in 1828, a “highest decree” was widely published regarding state-owned factories, which read: “To encourage the discovery of diamonds, decent monetary rewards should be established for those who will find this precious mineral in the districts of state-owned factories.” In 1888 and 1895 At the Krestovozdvizhensky mines, special exhibitions of diamond crystals were organized in order to familiarize prospectors with the external features of this precious stone. In 1898, the former owner of the Krestovozdvizhensky mines, P. Shuvalov, invited the French engineer B. Boutan, who tried to introduce here the search methods used in the placers of South Africa, as well as the systematic excavation of the placer, depositing the washed material and dismantling the concentrate on tables. Later, in 1902-1903, exploration for diamonds was once again carried out at the Adolfovsky and Krestovozdvizhensky placers with the removal of ore from the washed material. However, the work carried out did not produce positive results. In other regions of our country, isolated finds of diamonds were known in the Yenisei taiga (along the Melnichnaya River and Tochilny Klyuch) and on the Kola Peninsula (along the Paz River). In 1936, indications were received of the diamond potential of the Eastern Sayan, where microscopic fragments of diamond were recorded in the bedrock—in carbonaceous peridotite, but were later not confirmed. According to literary data, during the period from 1829 to 1937, 270-300 crystals were found in Russia, and 250 crystals were discovered on the western slope of the Middle Urals. However, industrial diamond deposits were not found in any area. The reasons for this failure are obviously that geological, prospecting and exploration work was carried out on a small scale; The indigenous sources of diamond placers were not reliably known, and the views of scientists on the issue of the origin of diamonds in indigenous deposits were very different; there were no sufficiently reliable methods for searching, exploration, testing and identifying diamonds in exploration samples. A new period in the history of diamond research in our country began in 1938. Since that time, prospecting and exploration work for diamonds has been carried out on a large scale. For these purposes, many geological organizations and research institutes of the country were involved. A number of institutes have begun to develop methods and technologies for the enrichment of diamond-bearing rocks. As a result of prospecting work carried out in 1938-1939, a number of diamond-bearing placers were discovered in the Middle Urals, in the lower and middle reaches of the river. Koiva and in the middle reaches of the river. Vizhay. Industrial diamond mining in the USSR began in 1941. As a result of geological prospecting and exploration work in 1941-1945. A number of new deposits were discovered in the Middle Urals. However, all of them were characterized by low diamond content and small reserves. Therefore, there was a need to strengthen geological prospecting work in the Urals in order to search for richer deposits, to organize scientific and geological prospecting work in new areas of the country. To accomplish these tasks, prospecting and exploration work in the Urals was significantly expanded and diamond searches were organized in the Yenisei Ridge, in the Eastern Sayan, in the basin of the Angara and Podkamennaya Tunguska rivers, on the Kola Peninsula, in the Far East, in Eastern and Western Siberia and in the North Caucasus . At the same time, diamond mining was developing in the Urals, for which new enterprises were built, more productive extraction methods were developed and improved. However, the indicated rates of development of geological exploration and production work turned out to be insufficient for a sharp increase in production. The first information about diamond finds in the river basin. Vilyuy in Yakutia was reported by the Yakut local historian, self-taught geologist Pyotr Khrisanfovich Starovatov. In his article “Mineral riches of the Vilyui River basin.” Before the revolution of two very valuable stones on the rivers Chone and Kempendyae. One miner was panning for gold on Chon. In a shallow place he saw a stone, the play of which in the sun attracted his attention. A gold buyer who came from the city of Olekminsk exchanged this stone for one and a half pounds of tobacco. The next year, the buyer again came to the same place and began asking about the prospector from whom he bought the stone. The prospector was no longer here. The buyer was asked: “Why are you looking for this prospector?” “I sold the stone I received from him at a very high price, I would like to add,” was the answer... The second incident occurred at the Kempendyai resort with a certain Isaev, who profitably exchanged one stone for goods that were very valuable at that time. In this article, Starovatov does not call the “valuable stones” diamonds, but apparently they were. According to Yakut local historian Modest Krotov, who studied Starovatov’s archive, it is known that in September 1939, the Central Geological Exploration Organizations had already received from Starovatov specific information about the finds of diamonds in the river basin. Vilyui; this information is based not on oral accounts of eyewitnesses, but on Starovatov’s own findings. So far, nothing has been mentioned about Starovatov’s activities in the literature on Yakut diamonds. It was first mentioned by Doctor of Technical Sciences N.V. Chersky in his book “The Wealth of the Subsoil of Yakutia”. Meanwhile, Kh. Starovatov is essentially the first person to point out the presence of the river in the basin. Vilyuy diamonds. In 1949, diamonds were discovered in Yakutia along the river. Vilyui, in connection with which the center of geological exploration was moved to the Siberian platform. In 1950, diamonds were found in the river valley. Marzhi, and in subsequent years, many diamond-containing placers were discovered in the Vilyui region: along the rivers Vilyui, Markha, M. Botuobiya, Daldyn, Tyung, Morkoka, etc. A remarkable event was marked in 1954, when the first kimberlite pipe was discovered, which turned out to be diamond-bearing. Subsequent exploration showed that the diamond content of this pipe was low and it turned out to be non-commercial, but the significance of this discovery is certainly great. The debate about the sources of Siberian diamonds ended, since everyone could see both the diamonds in the rock and the typical companion of diamond - blood-red pyrope. In addition, the kimberlites themselves - the source rocks of diamonds - were of great scientific interest. In the same year, rich diamond placers were discovered in the river basin system. Botuobia and especially along the river. Irelyakh.
In June 1955, rich primary diamond deposits were discovered simultaneously in two areas: in Maly Botuobinsky - the Mir kimberlite pipe and in Daldynsky - the Udachnaya kimberlite pipe, and since 1956, along with exploration, associated diamond mining has been successfully going on here . In 1957, experimental industrial diamond mining began at the Mir pipe. Placer and primary diamond deposits discovered in 1954-1955. in the Vilyuisky district of the Republic of Yakutia, are the largest deposits of world importance. On their basis, a diamond industry has been created, which fully meets our country’s needs for diamonds.