A rough diamond is a hard mineral of natural origin, without cutting or polishing, unsightly in appearance and with a complete lack of shine. Only complete and careful diamond processing makes it an expensive diamond. The answer to the question: how and with what are diamonds processed will help you understand the reason for the high cost of crystals.
About the characteristics
The appearance of the stones depends on the order in which the atoms are built, the crystal lattice of which looks like a cube with four carbon atoms in the central part and one at each vertex. Rough diamonds are very durable, which is due to their specific structure. In addition to pure carbon, the composition may contain iron, magnesium, and calcium in small quantities.
Uncut diamonds vary in:
- density;
- blossom;
- purpose - technical and jewelry;
- physical and chemical properties;
- sizes , depending on weight - they are small, large, medium.
An uncut, rough diamond has various shapes, but most often looks like an octahedron, cube, dodecahedron, including rhombic. There are cubic specimens, among which rarely come across those of jewelry interest. Some mined diamonds even resemble shapeless blocks.
The color range of the stone is varied. Mostly colorless and yellowish minerals are found, occasionally red, pinkish, brownish, grayish, blue, black and even blue. Green specimens are rarely found in nature. The difference between crystals and each other is not only the color or shape, but also the degree of transparency.
The shades depend on what inclusions and impurities the stone contains, what it received under natural conditions of radiation exposure and structural features. Most minerals are unevenly colored, for example, one layer of color or several shades.
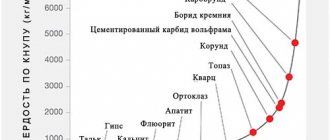
The hardness of diamonds received the highest, ten-point rating on the Mohs scale after comparison with other minerals. Their name translated from Greek means “invulnerable.” The rulers of Ancient Greece considered stones a symbol of power.
Diamond is an allotrope of carbon
Carbon itself is carbon. An element with atomic number 6 and a non-metal.
But when carbon bonds with other carbon atoms, it can create many structures, each with a unique set of properties. These different forms are called allotropes.
Allotropes are a feature of non-metallic elements such as carbon, silicon and phosphorus (which has as many as 6 allotropes).
Carbon has many allotropes. This is due to its valency. Carbon has four available electrons that it can share with other elements to create compounds. This valency gives it the unique flexibility to form different structures when combined with other carbon atoms.
Diamond has an octahedral structure, in which each individual carbon atom is attached to four other carbon atoms, forming a sort of three-sided pyramidal structure.
Notice how the topmost carbon bonds in the diamond look like a three-sided pyramid.
Other carbon allotropes form sheets (graphite and graphene), spheres (buckminsterfullerene), and even some strange nanostructures.
Features of stones
Each pebble has its own characteristics, called “inclusions”. It is they who give it uniqueness and difference from its peers.
Inclusion is considered:
- the presence of voids inside;
- inclusions of foreign substances;
- cracks.
Many people are interested in the question of how diamonds are processed. The stones are so strong that they can only be cut using the same stones. Diamond is called the hardest, but at the same time fragile mineral of natural origin. It breaks easily when dropped. It can be set on fire using an ordinary magnifying glass. The combustion temperature is 700°C and above.
Strong acids are not capable of harming the mineral and deteriorating its appearance, structure, or color. Soda and saltpeter, or more precisely, their alkaline solutions, have an oxidizing effect on diamonds.
The gem has luminescent properties, which turns it into an indicator that determines the presence of radioactive particles. Their presence is indicated by electrical impulses and light flashes.
Brief characteristics: diamond, graphite and coal
The crystalline lattices of graphite do not have strong bonds; they are separate flakes and seem to slide over each other, easily separating from the total mass. Graphite is often used as a lubricant for rubbing surfaces.
Coal consists of tiny particles of graphite and the same small particles of carbon, which are combined with hydrogen, oxygen, and nitrogen.
diamond crystal lattice is rigid, compact, and has high hardness. For thousands of years, people did not even suspect that these three substances had anything in common. All these are discoveries of a later time.
Graphite is gray, soft, and greasy to the touch, not at all like black coal. Outwardly, it rather resembles metal. Diamond is super-hard, transparent, sparkling, and in appearance is completely different from graphite and coal (more details: How minerals are used). Nature did not give any signs of their relationship. Coal deposits have never coexisted with graphite.
Geologists have never discovered sparkling diamond crystals in their deposits. But time does not stand still. At the end of the 17th century, Florentine scientists managed to burn the diamond. After that, not even a tiny pile of ash remained. The English chemist Tennant, 100 years later, found that when equal amounts of graphite, coal, and diamond are burned, the same amount of carbon dioxide is formed. This experience revealed the truth.
A little about mining
Diamonds are mined in Russia, Congo, South Africa, and Namibia. There are small deposits in Canada, Australia, Angola, and Botswana. Diamond mining is an expensive and labor-intensive process. Until the middle of the last century, the place to search for gems was mainly secondary deposits, the formation of which occurred as a result of the destruction of rocks. The process looked like gold mining: sand from the river was placed in a sieve using shovels and sifted, and then washed.
The largest reserves of future diamonds lie in primary rocks called “kimberlite pipes.”
Preparation for production is carried out in two stages:
- Formation of a 600-meter deepening using explosives.
- Laying mines.
Ore mined from the depths of the earth is delivered to factories for grinding and washing. To obtain one carat of crystals, sometimes it is necessary to process up to hundreds of tons of rock. Then culling takes place to select a certain type of diamond using water and X-rays, after which the stones are handed over to the cutters.
Precious mineral deposit
In ancient times, diamonds were mined by hand from the ground or river sediments.
According to legend, birds were used to extract gems from deep crevices. To do this, they threw pieces of meat into the gorges, which the eagles hunted for. The stones stuck to the pieces, then people took them from the birds or looked for them in the droppings. In this way they managed to find even large nuggets .
Be sure to see: Origin, properties and compatibility of aquamarine
In the mid-19th century, kimberlite pipes were discovered - these are pipe-shaped channels in the earth's crust that were formed during the breakthrough of magma. They are part of ancient volcanoes, the ground part of which has been destroyed. This depression is filled with igneous rock containing diamonds. According to experts, about 90% of all reserves of gems in nature contain kimberlite pipes. They got their name from the village of Kimberley (South Africa), where they first found such a crevice and crystals in it.
The main diamond mining centers are located in the Russian Federation (Sakha Republic), South Africa (Namibia, Botswana, Angola), Brazil (Minas Gerais, Bahia). You can also find gems in Australia, the USA, Canada, and Kazakhstan. Indian diamond mines are almost exhausted.
The world diamond market is led by the three largest in South Africa, ALROSA in the Russian Federation, and Rio Tinto Group in Brazil.
Quality and shape
According to statistics, only 20-25% of the raw materials mined in the world are used by jewelers. Only high-quality rough diamonds with impeccable color and clarity become part of precious jewelry.
Crystals with obvious defects, inclusions or foreign impurities are considered “technical”. Pebbles of poor quality and ugly color are needed in industry. In the same category there are gems of irregular shapes and too small sizes. They are used to make diamond dust, that is, they are crushed.
It is known that a diamond is a processed diamond. Its final form depends on the natural one acquired in nature. Uncut diamonds look dull and unattractive. The cutting process makes it sparkling, smooth, and expensive. Read more about the difference between a diamond and a diamond →
Without processing, the mineral is not particularly valuable, and they ask for no more than a hundred dollars. But a diamond made from diamond costs 4-10 times more.
The cost is also affected by the type of cut, which can be:
- round;
- fantasy.
Before processing, an oblong diamond takes on shapes called:
- marquis;
- drop/pear;
- oval;
- heart.
Stones, whose natural appearance had almost ideal outlines, receive one of the following forms:
- emerald;
- usher;
- radiant;
- princess.
Processing diamonds into round brilliants is a labor-intensive process that requires strict adherence to proportions. This causes the high cost of the round product. Learn more about how many facets a diamond has →
Magic properties
Diamond has outstanding magical abilities. Jewelry with stones cleanses the aura and gives vitality. This mineral loves generous, bright, worthy people. It enhances the good qualities of the owner and punishes him for the bad ones. Therefore, the stone is suitable for people who have a pure soul. Diamond products help build a personal life, as well as a brilliant career.
Since ancient times, diamonds have been used as an amulet, because they protect against magical influence. The Egyptians believed that the gem could protect the owner from the effects of toxic substances.
Modern people wear a diamond ring or bracelet on their left hand to attract true love. Yellow colored stones are used for various rituals. The white crystal is a strong amulet against black magic. For your business to flourish, you need to wear jewelry with a diamond in a gold setting.
How diamonds become diamonds
The gems to be processed must initially be of good size. The future diamond, that is, an uncut diamond, weighs 40-60% more than after finishing work on its creation.
People learned to work with precious stones long ago, but the stubborn crystal succumbed to them only in the 15th century. Diamond processing has always been a painstaking task, requiring several stages, during which numerous methods of work were tried.
Diamond uncut:
- polished by rubbing one stone against another;
- hammered into crumbs used to coat metal discs;
- sawed;
- acquired edges and planes in a certain quantity.
So why don't diamonds turn into graphite?
So, if there is a more stable form, why haven't all diamonds turned into graphite? For two reasons.
Firstly, diamond is stable under the conditions existing on Earth. Additionally, graphite is only a few electron volts more stable than diamond (on Earth). The difference in stability between diamond and graphite on Earth is not that great.
Secondly, it takes a lot of energy to convert diamond into graphite.
In other words, the energy required to move a diamond from the well to the valley floor, where it turns into graphite, is very large.
Chemists and geologists tried to turn diamond into graphite. They discovered that when a diamond is compressed with an indenter (basically a sharp object that can be used to pierce the diamond), the surface of the diamond in contact with the indenter turns into graphite.
If squeezing a diamond isn't your style, scientists have also found that low pressure and very high temperatures (1,500 to 1,900 degrees Celsius or more) will work. Add iron to the mixture and it will speed up the process (called graphitization).
Diamonds also have their weakness
However, do not subject diamonds to high pressure if you want to turn it into graphite. Diamonds are more stable at high pressure than graphite, which is why they form in the Earth's mantle (and even sometimes on asteroids). However, under certain conditions, diamonds can turn into graphite even under high pressure.
Diamond Processing Methods
The question of how diamonds are made has two answers: manually and using a laser.
How to make a diamond from a diamond by hand:
- Splitting. Following the lines that were made by the specialist during the inspection, small cuts are made on the stone placed in the holder with the same mineral. Afterwards a split occurs with a blow.
- Sawing. At this stage, the stone is attached using limestone or gypsum to a copper head, which is clamped in a special cutting tool. For cutting, a thin disc is used, lubricated with oil mixed with diamond powder. The process speed is approximately 1 mm/hour.
- Adding roundness. The mineral becomes round, making it look like a diamond. Processing is carried out using another stone.
- Cut. The crystal is fixed in the grip of the grinding machine, the quadrant, so that a precise angle is obtained in relation to the grinding disc for applying the bevels. The discs, usually steel, are lubricated with a special paste or oil mixed with diamond powder.
Technologies are constantly improving, new ones are replacing old ones. This is why some diamonds are laser cut.
When choosing this method, each stage of the formation of the future diamond occurs using laser systems. A crystal classified as jewelry is assessed by a specialist who determines the processing method. The cutting lines are drawn using a laser. Then comes the turn of cutting and cutting, naturally, with a laser.
Laser processing allows you to give stones the desired shape without taking into account their direction when fixing. The negative point is significant loss of diamond mass, which does not happen when processing manually. About the weight of a diamond →
Despite the attempt to make working with precious stones easier, only a talented craftsman can create a masterpiece from them, and only with his own hands. Usually several people work with one stone at once. Each of them is involved in a certain stage, and the two of them work together to shape the diamond.
Hard, shiny and almost eternal
We loved and love the word “most”. The fastest, the smartest... If you ask what is the hardest, most likely we will get the answer: diamond. This answer is almost accurate. Why is diamond even the most brilliant? And at the same time, is he really the best?.. This is what we will talk about.
Cutting a diamond from a natural crystal and the path of rays. Drawing: Zoya Florinskaya.
Photo: Kiyoshi Takahase Segundo / Lori Photobank / PantherMedia.
Evolution of cutting. Drawing: Zoya Florinskaya.
Names of faces. Drawing: Zoya Florinskaya.
Science and life // Illustrations
South African 10 rand banknote. African white rhinoceros. Cut diamond. Photo by Evgeny Mukhortov / Lori Photobank.
‹
›
Shiny pebbles were found on the territory of modern India seven thousand years ago. People were amazed by their brilliance, hardness, and sometimes transparency (it was a long time before the invention of glass). The name of the mineral comes from the ancient Greek “adamas” - indestructible. Since ancient times, diamonds - cut diamonds - have been a symbol of wealth and power; they were used to decorate scepters and crowns, statues and priestly robes.
In ancient times, incredible properties were attributed to diamonds; Pliny the Elder, who gave the first detailed description of a diamond, could not resist fantastical inventions. In his work “Natural History of Fossil Bodies,” he wrote that diamond neutralizes the effects of poison and frees people from empty fears. There was also a belief that a diamond could be dissolved in fresh goat blood. It was believed that if you put a diamond crystal between a hammer and an anvil, the tools would most likely shatter into pieces, but the “king of stones” would remain indestructible. Such an illiterate test destroyed many good stones, because hardness and impact resistance are different properties.
Among all the diamonds found, and their total weight to date amounts to tens of tons, regular transparent and colorless polyhedrons are rare. Most often, a large, irregularly shaped diamond is not much different from a cobblestone. This is exactly what the largest diamond ever found, the Cullinan, looked like. It was discovered on January 26, 1905 in South Africa in the Premier mine and named after the company's president, Sir Thomas Cullinan. The fist-sized stone weighed more than 600 grams, and judging by its shape, it was once part of a larger formation. When it was delivered to King Edward VII of Great Britain, he admitted: “If I had come across this stone, I would have contemptuously kicked it away.” The Cullinan lived outside the mine for only three years - the miracle of nature was split and sawed to make 9 large diamonds and 95 smaller ones.
A rough diamond, even of the correct shape, really looks inconspicuous: just a very shiny piece of glass. Only cutting turns it into the “king of stones”. But this process is very difficult due to the hardness of the stone. Mechanically, only another diamond can “take” it, and the processing can last for weeks and months. Natural diamonds were probably first processed in ancient India. But they did it very primitively: until the 15th century, everything came down to light grinding and polishing of natural edges so that they would shine better. In Europe - at the French and English courts - octahedral crystals were called diamond tips, and they actually sometimes resembled the tip of a spear. Imagine two small tetrahedral pyramids folded at their bases - this will be an octahedron.
From diamond to diamond
Gradually, craftsmen learned to give diamonds different shapes, sometimes absolutely fantastic. But at first they had no idea what miracles were hidden in the stones - the beauty of a diamond is revealed only when cut correctly. Slowly, over centuries, masters groped their way to learning how to reveal the unique properties of diamonds. Experience was accumulated in the processing of not so hard minerals - rubies, emeralds, topazes. In the 13th-14th centuries, grinding mills appeared in Europe - large stone grinding discs rotated using a water drive. An important step forward was the use of the finest diamond powder for grinding. It was pressed into a metal disk to create a grinding wheel, and this made it possible to significantly increase labor productivity.
It is believed that the first diamond was made in Paris at the beginning of the 17th century. And for several decades the city remained the world's main diamond processing center. But gradually it lost the palm to Antwerp and Amsterdam. In the mid-19th century, 540 steam-powered grinding machines were already operating in five workshops in Amsterdam. In the 20th century, thousands of workers also cut diamonds at factories in Germany, and after the war - in Israel, India and (on a smaller scale) in the USA and South Africa. However, to this day, jewelers have retained French terms: “pavilion” (French “tent”) - the lower part of the stone, “collet” (French “collar”) - the tip or small platform below; “facet” or “fatset” (French “edge”) - a beveled part of a sharp edge or edge; and the word “diamond” itself comes from the French “brilliant.”
Many methods have been developed for cutting diamonds. At the beginning of the 15th century, the production of so-called thick diamond tables began - one of the vertices of the octahedron was cut down to form a wide square face and the opposite lower vertex, the colette, was slightly blunted. Later they began to make “thin stones” - the two opposite vertices of the octahedron were severely sawed off.
But a real diamond was created from a diamond only when the craftsmen used facet cutting. It consisted of applying symmetrical small edges - facets - to the crystal. At first, eight facets were applied to the upper and lower parts of the “thick stone” (jewelers call this cut simple or ordinary). Further complication of the cut led to the application of additional 36 facets - 18 each on top and bottom. This is the so-called double cut, or “Mazarin cut” (named after Cardinal Jules Mazarin, who did not cut diamonds himself, but collected a rich collection and bequeathed it to the French crown). Further - more: at the end of the 17th century, the triple “Peruzzi cut” (named after the Venetian Vincenzo Peruzzi) appeared - with 58 facets.
Over time, other types of cuts became widespread: many small triangular facets for an old Indian rose, a full Dutch rose has 24 triangular facets; the half-Dutch cut has 16 facets, the double Dutch cut has 36, and the simpler Antwerp cut has 12. The cross rose has a more complex cut: 16 of its 24 facets are quadrangular, the rest are triangular.
What all of the above “roses” have in common is a flat base, above which a shiny “tent” rises.
In the 19th century, the most common cut was the so-called old brilliant cut, which existed until 1910. It comes very close to what can be considered modern - it is also called a “full round diamond”. In it, the angles between the facets are slightly changed, and the thin belt (girdle) separating the upper and lower parts of the stone is made perfectly round; the height of the upper part of the stone - the crown, on which there are at least 32 facets, has been reduced, while on the lower part - the pavilion - there should be at least 24 of them.
Nowadays, cuts of three very similar types are mainly used. The “American Diamond” was theoretically calculated by Marcel Tolkowsky in 1919, and this cut is adopted in the USA and Great Britain. The “Practical Diamond” by Werner Eppler was developed in 1939; the cut was adopted in Germany, Russia and the CIS countries. The name is due to the fact that in order to determine the optimal angles of inclination of the facets, instead of theoretical calculations, mass measurements were made of the most beautiful diamonds, made at different times by different masters and having the maximum play of light.
Finally, the “standard Scandinavian diamond” adopted in the Nordic countries was developed in 1968. Its proportions and angles, like those of the Eppler diamond, are not calculated, but are found empirically, by measuring the most spectacular diamonds.
How different are these three types of cut? The angles of inclination of the facets of the crown and pavilion relative to the upper platform (table) and lower colette are 34°25′ and 40°46′ for the Tolkowsky diamond; 33o12′ and 40o48′ for the Eppler diamond; 34o30′ and 40o45′ for a Scandinavian diamond. To visualize such precision of installation of the grinding disk, it must be taken into account that for a circle with a diameter of 10 cm, the movement of the edge of the disk for 1 minute (1′) is 0.015 mm, 15 microns, a third of the thickness of a hair.
The cut depends on the optical properties of the diamond, specifically on the refractive index. And it depends on the impurities that are always present in a diamond; By the way, other properties, such as color and electrical conductivity, also depend on them. The set of diamond samples that was used to determine the optimal cut (directly or through calculation of the average refractive index) varied among different authors. And therefore the results obtained differed slightly.
In addition to the main types of cuts, there are many others that are applied to the largest stones. Thus, the “Highlight” cut has 73 facets, the “Cara star” - 74, the “anniversary” - 88, the “magna” - 102, and the “princess-144” cut, developed in 1965, has as many as 146. But if you buy a ring with a small diamond, then it will most likely have a figure-of-eight cut - eight facets in the crown and pavilion, and in total, including the table and tunic, 18 facets.
What did jewelers and mathematicians strive for, precisely, down to hundredths of a degree, when calculating the angles of inclination of the edges, checking the height of the crown and pavilion, and determining the thickness of the girdle? Everyone pursued one goal - to maximize the “internal resources” of the stone. And this means making the play of light highlights as bright as possible (with minimal loss of weight of the natural crystal). Moreover, the latter factor often “outweighs” the inevitable loss of mass, which during cutting can amount to more than two-thirds of the original stone (!). Moreover, owners of old stones often give them to jewelers for recutting, despite the loss of 30-35% of the mass.
“To be a hundred times more expensive...”
The classical diamond processing technology is as follows. First, all diamonds are examined to determine the processing method and type of cut. Then this diamond is split (if necessary), sawed, followed by turning, cutting, grinding and polishing. If a diamond has any defect (crack, dark spot), then they either cut along the defective area, or try to isolate the area with the defect into a smaller crystal in order to obtain a high-quality diamond from the remaining part. Sawing is carried out with thin disks made of bronze (an alloy of copper and tin), into the surface of which diamond powder is pressed. Disc thickness is 50-90 microns, rotation speed is 3-15 thousand rpm. Diamonds are cut in the direction of the least hardness of the crystal (in the direction of the greatest hardness, diamond is almost impossible to process). Then the turning begins. Losses during this operation are 15-25%; but the waste is used to make diamond powders. Then the cutting of the stones begins, followed by polishing (it is carried out using cast iron discs, into the surface of which diamond powder is also pressed). The rotation speed of the cutting disk is 2500-2800 rpm, with each stage the powder becomes finer, and during final polishing the particle sizes are reduced to 3-10 microns.
Cutting and polishing are the most labor-intensive operations. For example, at Belgian enterprises, out of 120 workers, one is engaged in chopping diamonds, six are engaged in turning, and the rest are engaged in cutting, grinding and polishing. Naturally, technological processes are being improved, and semi-automatic machines have been created for cutting. In addition, other diamond processing methods are being developed, of several types. Firstly, associated with chemical reactions, since diamond reacts with some substances at elevated temperatures - even at a decent speed. Secondly, carbon diffuses into metals, some especially readily (for example, into iron and at elevated temperatures). Therefore, if you press iron onto a diamond and heat it, carbon from the surface of the diamond will go into the metal. Thirdly, diamond can be processed in a vacuum - a stream of accelerated ions that spray the surface. Finally, the diamond can be treated with a laser beam - which, depending on the environment, heats it until it oxidizes or vaporizes it.
However, what are all these tricks for?
"Multi-colored lightning"
Of course, the diamond first attracted attention as a piece of jewelry. A properly cut diamond, that is, a diamond, divides the light falling on it into many separate rays. The higher the refractive index of light, the more complex the cut can be (to achieve maximum effect). For diamond it is approximately 2.4; There are very few substances with higher refraction, and not all of them are sufficiently transparent. That is, diamond has practically no competitors in this parameter.
Moreover, another optical property is important - dispersion, when light of different colors in the same substance has a different refractive index. Rainbows and colored “bunnies” on the wall when sunlight hits the edges of mirrors and other glass are the result of dispersion. It is quantitatively determined by the difference between the refractive index for violet and red rays. And the greater this difference, the wider the rainbow stripe will be, the more the crystal will shimmer at the slightest turn. For diamond, this value is 0.044; substances with greater dispersion, again, are very few. Moreover, substances with higher refraction tend to have low dispersion, and substances with high dispersion tend to have low refraction. Therefore, diamond, without being an absolute record holder in any of these parameters, wins in the “all-around”. More or less similar in properties to diamond are moissanite SiC and cubic zirconia - ZrO2 with the addition of oxides Hf, Mn, Ca, Y, and they are actually used as cheap imitations of diamond.
Jewelry is usually viewed in reflected light, so the diamond's facets must be positioned to fully reflect as much of the light falling on the stone as possible. In a properly cut diamond, a ray of light, falling on the upper platform and facets of the crown, passes into the crystal, is completely reflected from the facets of the pavilion, makes its way back and exits through the crown - preferably through its inclined faces. If you look at such a diamond through the light, turning the table (upper platform) towards the light source, then it will be completely dark, since all the light falling on it will come out in the opposite direction. Passing its path inside the diamond, refracting at its external and internal edges and reflecting from them, the light is decomposed into a range of colors, which gives that beautiful rainbow play that is characteristic of a diamond. A simple reflection from the upper faces is added to it - it gives the so-called diamond shine. And the lower edges, with full internal reflection of the rays, seem to be silvered and have a metallic sheen. All this creates that unique “play” of light, that “fire” that makes a diamond a diamond. As the stone connoisseur, President of the British Gemological Association Herbert Smith wrote, at the slightest movement of a cut diamond, “the constantly changing shades attract the eye: first, the diamond sparkle of the purest white color and immediately the azure-blue shine, turning from waxy to exquisite orange and fading into crimson flickering..."
"Guardian of eternal purity..."
For science and technology, azure blue and exquisite orange are not the most important colors. Diamonds have been used in technology for a long time and widely - if you can grind and polish the diamond itself with diamond powder, then all the more so can everything else. Indeed, diamond powders were used to process everything in the world, and diamond crystals were used to cut, for example, glass. They were used so widely that the name “diamond” was assigned to a tool for cutting glass and was used even when the cutting element was not a diamond, but another (but also very hard) material. At first, diamond could not be used particularly widely - after all, it was expensive, like waste from cutting diamonds. But over time, people mastered the synthesis of diamonds at high pressures and temperatures, and even later they learned to spray diamond films onto the surface of the tool, thereby increasing its wear resistance.
Another quality of diamond that people are now looking for is its high thermal conductivity (it is several times greater than that of copper). This is very important for semiconductor technology, since the operation of semiconductor circuits is accompanied by the release of heat. If it is not removed, the temperature will begin to rise and the device will fail. To remove heat, it must be “pulled” through the volume of the device and the substrate, which separates the device itself from the coolant flow (air or water). Moreover, this substrate must be an electrical insulator - and this is sad, because insulators, as a rule, conduct heat poorly. So, diamond simultaneously acts as an insulator and at the same time conducts heat well. The question arises: is it possible to make the devices themselves from diamond? It's very possible. But to do this, you need to learn how to grow diamonds that would not be insulators, but semiconductors, and of both types - p-type and n-type. In principle, this problem is solvable, that is, it is known that it can be solved. Moreover, it seems that a sample pn junction already exists. It is possible that we are witnessing the emergence of a new direction in semiconductor technology. And not only there - diamond will also find its application in powerful vacuum electronics. And powerful vacuum devices include radar, long-distance communications, and tokamak...
In science, high compressive strength is used: to make “anvils” from diamond - the very elements of the installation through which ultra-high pressure - millions of atmospheres - is transmitted to the object. Before that, all research in the field of high pressures “rested” on these “anvils” - and this is the path to hypothetical metallic hydrogen, and much more!
Are they really eternal?
Geologists have always been interested in the question of how old a diamond is. The radiocarbon method, developed by Willard Libby in the middle of the last century, could not give anything: the carbon isotope C-14 has a half-life of 5730 years, that is, in a million years not a single atom will remain of it. And relatively recently, American geochemists were lucky: they had at their disposal South African diamonds that contained garnet inclusions - beautiful small crystals. In general, garnet is the collective name for a group of silicates of complex composition, which includes one and a half dozen individual minerals (among the known ones are grossular, pyrope, and uvarovite). In addition to silicon and oxygen, they contain ions of Ca, Fe, Mg, Mn, Al, Cr, Ti, V, Zr. All this would have been of little help in determining the age of the mineral, but, fortunately, it contained small impurities of samarium. This rare earth element occurs in nature in seven stable isotopes, two of which are actually slightly radioactive, but have gigantic half-lives: 106 billion years for samarium-147 (about 15% of it in the earth’s crust) and 7 quadrillion years for samarium-147. 148 (his - 11%). The second isotope is of little use due to its too long lifetime, but the first one turned out to be what was needed. Slowly decaying, it turns into neodymium-143. By the amount of this neodymium, knowing the rate of its formation, one can determine the age of the sample, that is, the time when samarium got into it and began (or rather, continued) to slowly decay. For example, it is obvious that in 106 billion years the number of atoms of neodymium-143 and samarium-147 will be equal. It turned out that not much neodymium had accumulated in the garnet. This means that samarium entered the garnet 3.2–3.4 billion years ago; Some of the uncertainty stems from the inaccuracy of detecting minute traces of samarium-147 and even more minute amounts of neodymium-143. And if the Earth is now about 4.6 billion years old, then the diamond - at least this sample - is not much younger than it.
Whether this can be considered an eternity is up to us to decide. But what is certain is that in the coming decades, science and technology will increasingly use diamonds.
By the way, about “the hardest”. Doubts have already arisen about this: some groups of researchers claim that using fullerenes (carbon again!) it is possible to make a material harder than diamond. Others write that this is impossible. It is possible that neither this nor that is true—the concept of “hardness” just needs to be clarified. However, in science it is constantly necessary to clarify definitions from time to time.
About fakes
Attempts to create an artificial diamond began in 1797, but they were crowned with success only in 1956. Over the decades, technology has improved so much that it can be difficult to distinguish an artificial stone from an original one. Some imitation diamonds are so beautifully crafted that only those who know what a real diamond looks like can tell the difference between them and the original.
The most common “fake” is called cubic zirconia. The second stone that imitates a crystal of natural origin is moissanite, which can only be distinguished by those who know how to verify its authenticity. The third option is asha. What gives it its shine is a layer of carbon atoms, that is, what a real stone is made of, which makes identification by eye a difficult task.
Growing artificial diamonds using high temperatures and pressure, invented in the 1950s, produces almost natural crystals. This is explained by the fact that natural stones appear under similar conditions, but over a longer period.
Pebbles that do not have time to go through the full growth cycle when they hit the earth's surface require additional exposure to temperature and pressure in laboratory conditions. This allows them to become full-fledged diamonds, slightly “modified” by humans. After additional procedures, they become completely ready to be turned into a diamond.
How to distinguish a fake and purchase a natural stone
Diamonds have more copies than other precious stones. You can distinguish a diamond from cubic zirconia or a simple piece of glass in the following ways:
- Look through the crystal at the sun. Through natural stone you can only see a bright point, but imitation transmits light completely.
- Place a diamond in water: a real diamond will sparkle, but a glass fake will not.
- Moisten the crystal in water, and then run an aluminum stick over its surface. There will be a silver stripe on the counterfeit, which will be difficult to get rid of, but there will be no traces left on the original.
It is very difficult to distinguish a natural diamond from a synthetic one, since the technologies for creating artificial stones are constantly being improved. The first thing that should alert the buyer is the low price.
Only a gemologist (a professional gemstone appraiser) can give a 100% guarantee of the authenticity of a diamond. Using the tips described above, you can only identify low-quality imitation.
Authentication
Sometimes the question arises about how to check a diamond for authenticity. After all, its high cost is an excellent reason for creating fakes and various imitations passed off as a real crystal. You can do this with the help of a specialist or yourself, at home.
How to determine the authenticity of a diamond:
- According to rudinist - a narrow boundary dividing a faceted crystal into upper and lower parts. It should be matte. Transparency indicates artificial origin.
- Hardness. Real diamond leaves marks on glass surfaces. It also scratches other minerals such as sapphires and rubies. The only exception to this method is moissanite, which has a hardness similar to diamond.
- Glitter and refraction of light. A real diamond sparkles, but not as much as moissanite. A natural crystal differs from fiant and zircon in its light refractive index: if you place the stones on a printed text, for example, a page of a book, you will not be able to see the letters through the original.
- Defects and inclusions. They are present in real stones and are absent in fakes, but in no case are they cracks on the surface, scratches or chips. What is diamond clarity →
- Light scattering and ultraviolet. A beam of light directed through a fake will remain just as intense. A real diamond glows under ultraviolet light.
- Marker drawing. A line drawn with a felt-tip pen or marker on the surface of a gemstone will be clear and even, whereas on a fake it will be blurry.
- Exposure to acids. Submerged in an acidic solution, a real diamond will endure the test with dignity, emerging unharmed.
- Indelible. It is difficult to erase a real stone, so you will have to examine the edges of the stone that has raised doubts. If they are smoothed out and appear erased, it is a fake.
Diamond rightly deserves the title of a unique and irreplaceable stone in the industry. At different times it was used for various purposes, but only when it gained jewelry interest did it become truly expensive. Its cost depends on the processing method, shape and changeability of fashion, but demand always remains high and is unlikely to ever change.
Graphite, not diamond, is the most stable allotrope of carbon.
Although diamond's tetrahedral structure makes it the hardest substance known to mankind, it is not the most stable form of carbon.
This title belongs to graphite.
You see, diamond is a metastable structure of carbon. Metastable in chemistry means that the structure is more or less stable under certain conditions, but there is an even more stable state.
A common analogy is to imagine a ball rolling down a valley. The most stable place for the ball will be at the bottom of the valley (as seen in the deeper valley in the picture below).
Now imagine the ball getting stuck in a smaller hole (a smaller valley in which the ball can be seen).
The ball is stable in the smaller well, but since the well is higher compared to the valley floor, this is not its most stable state. But the ball will remain there unless work is done to pull the ball out of the well to the bottom of the valley.
Understanding the stability of chemicals.
Our diamond is like a ball in a well, and our graphite is like a ball at the foot of a hill.
In terms of chemistry, diamond is kinetically stable because it is trapped in a well, whereas it is thermodynamically unstable because there is a more stable form of graphite that it can transform into under the right conditions.