Graphite is one of the minerals that is distinguished by its versatility in practical use. It is usually customary to associate it with coloring substances, but its capabilities are not limited to this. At the same time, we cannot talk about the universality of use of this material, since its layered structure also determines the scope of application. And this is not to mention the need to create special processing conditions. The fact is that the melting point of graphite in degrees Celsius can reach 2800 °C, which requires the use of special facilities for the manufacture of final products.
Characteristics of graphite
Graphite is a representative of the class of native elements of high strength.
Its structure has a large number of layers. There are two types of graphite found in nature:
- coarse crystalline,
- fine-crystalline.
Based on the size of the crystals and their location relative to each other, the following types of graphite are found in nature:
- clearly crystalline,
- cryptocrystalline.
Graphite has a fairly layered structure. Each layer has a wavy shape. It is mild.
Graphite is one of the elements that consists mainly of crystals of different sizes. They have a plastic structure and small scales along the edges. In terms of their strength, they can be compared to diamonds.
The crystal lattice of graphite consists of a large number of layers, which have different locations relative to each other.
Today, artificial graphite is often produced, which is created from a mixture of various substances. It is used in various sectors of human life. Artificially produced graphite has a large number of types.
In the modern world, it is planned to extract gold from graphite. Scientists have found that one ton of graphite contains approximately 18 grams of gold. This amount of gold ore is inherent in gold deposits. Currently, it is possible to obtain gold from graphite not only in our country, but also in other countries of the world.
Basic properties of natural graphite
Graphites are substances of gray color with a metallic luster, amorphous, crystalline, or fibrous composition, greasy to the touch, specific gravity from 1.9 to 2.6. In appearance, graphite has a metallic lead-gray color, ranging from silver to black, with a characteristic greasy sheen. Therefore, consumers often call clearly crystalline graphites silver, and cryptocrystalline ones black.
Graphite feels greasy to the touch and gets dirty easily. On surfaces it easily produces a silver to black shiny finish. Graphite is distinguished by its ability to adhere to solid surfaces, which allows it to create thin films when rubbed against the surfaces of solids.
Graphite is an alothoropic form of carbon, which is characterized by a specific crystalline structure that has a peculiar structure.
Depending on the structural structure, graphites are divided into:
- clearly crystalline,
- cryptocrystalline,
- graphitoids,
- highly dispersed graphitic materials, commonly called coals. In turn, clearly crystalline graphites according to the size and structure of the crystals are divided into:
- densely crystalline (Bogotol graphite deposit),
- scaly (Taiginskoe graphite deposit).
In flake graphites, the crystals have the shape of plates or leaves. Their scales are oily, plastic and have a metallic sheen.
Graphite
Home / Cases / Graphite
Practical meaning:
Graphite is used very widely. We can say that there is not a single industry where it is not used to one degree or another. Graphite is needed mainly in the metallurgical industry for the manufacture of refractory crucibles and for coating the surface of foundry molds in order to protect the casting from burning of the molding earth; in addition, in the electrical industry - in the production of electrodes and arc carbons, in the production of pencils, black paints, black copy paper, printing ink or Chinese ink. It is also used as a lubricant (in cases where oil cannot be used due to high heat) and in steam boilers as an anti-scale agent. Recently it has been used for the manufacture of graphite blocks for “nuclear boilers” and for the manufacture of space technology. Artificial diamond is obtained from graphite. Graphite liquid is used for volumetric pressing of automobile parts. Dies coated with this substance ensure high surface cleanliness of steel workpieces, which eliminates their subsequent wiping on grinding machines.
Properties:
Conducts electricity well. Unlike diamond, it has low hardness (1 on the Mohs scale). Relatively soft. After exposure to high temperatures it becomes slightly harder and becomes very brittle. Density 2.08-2.23 g/cm³. The color is dark gray, metallic luster. Infusible, stable when heated in the absence of air. Greasy (slippery) to the touch. Natural graphite contains 10-12% admixtures of clays and iron oxides. When rubbed, it separates into separate flakes (this property is used in pencils).
Origin
Formed at high temperatures in volcanic and igneous rocks, pegmatites and skarns. It is found in quartz veins with wolframite and other minerals in medium-temperature hydrothermal polymetallic deposits. Widely distributed in metamorphic rocks - crystalline schists, gneisses, marbles. Large deposits are formed as a result of pyrolysis of coal under the influence of traps on coal deposits (Tunguska basin). Accessory mineral of meteorites.
Properties of the mineral
- Origin of the name:
from the Greek γράφω - I write - Year of discovery:
known since antiquity - Electrical properties of the mineral:
Conducts electricity well - Thermal properties:
Does not melt (burns at 3500 °C) - IMA status:
valid, first described before 1959 (before IMA) - Strunz (8th edition):
1/B.02-10 - Hey's CIM Ref.:
1.25 - Dana (7th edition):
1.3.5.2 - Dana (8th edition):
1.3.6.2 - Molecular Weight:
12.01 - Cell parameters:
a = 2.463Å, c = 6.714Å - Ratio:
a:c = 1 : 2.726 - Number of formula units (Z):
4 - Unit cell volume:
V 35.27 ų - Twinning:
by {1121} - Spot group:
6/mmm (6/m 2/m 2/m) - Dihexagonal Dipyramidal - Space group:
P63mc - Density (calculated):
2.26 - Density (measured):
2.09 — 2.23 - Specific gravity:
2,1 — 2,3 - Pleochroism:
strong - Type:
single axis (-) - Optical anisotropy:
extreme - Color in reflected light:
iron black fading to steel gray - Excretion form:
Leafy, scaly, radial, earthy aggregates - Classes according to the taxonomy of the USSR:
Non-metals - IMA classes:
Native elements - Chemical Formula:
C - Syngony:
hexagonal - Color:
Iron Black, Dark Steel Gray - Feature Color:
Black, Shiny - Gloss:
metallic matte semi-metallic - Transparency:
Opaque - Cleavage:
very perfect according to {0001} - Fracture:
mica-like - Hardness:
1 1,5 2 - Microhardness:
VHN10=7 – 11 - Literature:
Lobzova R.V. Graphite and alkaline rocks of the Botogol massif region.
Physical properties of graphite
One of the main properties of graphite is its ability to conduct electric current. Its physical properties differ from those of diamond in that it does not have such a high level of hardness. Its structure is initially quite soft. However, once heated, it becomes hard and brittle. The material begins to crumble.
The physical properties of graphite are as follows:
- does not dissolve in acid.
- Melting of graphite at temperatures below 3800 degrees Celsius is impossible.
- after heating it acquires a hard and brittle structure.
These are not all the properties of graphite. There are also parameters that make this element unique.
Graphite has the following characteristics:
- The melting point of graphite is 3890 degrees Celsius,
- graphite color is dark gray with a metallic sheen,
- The heat capacity of graphite is 0.720 kJ
- The resistivity of graphite is 800.000 · 10 − 8 (Ohm · Meter).
Attention:
The only parameter of all the characteristics of graphite that depends on the type of element is the thermal conductivity of graphite. It is 278.4 to 2435 W/(m*K).
Table. Physical properties of graphite.
| Characteristics | Flow direction | Temperature, °C | ||||
| 20 | 200 | 400 | 600 | 800 | ||
| Thermal conductivity coefficient λ, W/(m°C) of graphite: | ||||||
| - crystalline | || | 354,7 | 308,2 | |||
| - natural | _|_ | 195,4 | 144,2 | 112,8 | 91,9 | 75,6 |
| - pressed | || | 157 | 118,6 | 93,0 | 69,8 | 63,9 |
| - artificial with p=1.76 g/cm3 | _|_ | 104,7 | 81,4 | 69,8 | 58,2 | |
| - the same, with p = 1.55 g/cm3 | || | 130,3 | 102,3 | 79,1 | 63,9 | 53,5 |
| Tensile strength σpts, MN/m2 | || | 14,2 | 15,2 | 15,9 | 16,5 | 17,6 |
| _|_ | 10,3 | 11,3 | 12,0 | 12,5 | 13,7 | |
| Elastic modulus E, MN/m2 | || | 5880 | 7100 | 7350 | 7500 | 7840 |
| _|_ | 2700 | 3040 | 3200 | 3630 | 3920 | |
| Specific heat capacity s, kJ/(kg0C) | 0,71 | 1,17 | 1,47 | 1,68 | 1,88 | |
| Electrical resistance re104, Ohmsm | 16 | 13 | 11 | 10 | 9 | |
| Linear expansion coefficient α·106, 1/°С | || | 7,2*1 | 8,5*2 | 10,0*3 | 13,0*4 | |
| _|_ | 4,0*1 | 5,5*2 | 6,8*3 | 9,3*4 | ||
| || | 1,8*1 | 1,55*2 | 1,45*3 | 1,40*4 | ||
Reference material
Characteristics of graphite
Electrical conductivity
Graphite has good electrical conductivity. As temperature increases, electrical conductivity increases. In this regard, the temperature coefficient of resistance of graphite, unlike metals, is negative for graphite.
Specific Electrical Resistivity
Resistivity is a physical quantity characterizing the ability of a substance to prevent the passage of electric current. The higher the density of graphite, the lower the resistivity. Unit: Ohm*m
Thermal conductivity
The thermal conductivity of graphite is higher than that of many metals and decreases as the temperature increases. The thermal conductivity of graphite depends on the final processing temperature. Unit: W/(m*K)
Heat resistance
Graphite does not melt, but sublimates at a temperature of ~3900°K and withstands sudden temperature changes.
Wettability
Graphite is not wetted by most molten metals and molten glass.
Oxidability
In the presence of excess air, graphite begins to oxidize at a temperature of 750°K. Graphite is insoluble in solvents of organic and inorganic origin, and does not interact with many acids, alkali solutions and salts.
Mechanical strength
The tensile, compressive and bending strength of graphite increases as the temperature rises to 2700°C, and only then begins to decrease.
The mechanical strength of graphite is characterized by such values as bending strength
and Compressive Strength
. With increasing density, both of these quantities increase. Unit of measurement: MPa.
Purity
All graphites contain greater or lesser amounts of mineral impurities (ash). A special technology for cleaning graphite makes it possible to reduce the ash content to 10-4-10-5% by weight with an ash content of up to 0.5% in the starting materials.
Machinability
Graphite is easy to machine; its properties and structure make it possible to produce products of complex shapes, with small tolerances and with high accuracy. The combination of such a number of positive properties in one material predetermined its extremely wide application.
Anisotropy of physical properties
The physical properties of graphite depend on the orientation of the coke grains that form the recipe. In turn, the physical properties of graphite are influenced by the pressing method. Graphite, which is pressed using the extrusion method, is characterized by a clearly defined anisotropy of properties. The grains are oriented perpendicular to the direction of pressing. Graphites produced by stamping and isostatic pressing are more isotropic than those produced by extrusion.
ANISOTROPY OF PROPERTIES
Since the characteristics of different grades of graphite differ, they are influenced by the method of pressing, for artificial types - the orientation of the coke grains.
Graphite is quite plastic and easy to machine. Different types differ in the level of fat content, which is why it is used as a lubricant. It is necessary to note one more feature of pure types of this material: graphite has the highest moderation coefficient and low neutron absorption rate.
Applications of diamond and graphite
Both of these substances are used by humans both in industry and in everyday life. The characteristic properties determine the scope of application.
There are jewelry and technical diamonds. No more than 22% of gems are used in jewelry. For this purpose, the best, usually natural, stones are selected. They are cut taking into account the structure. All kinds of jewelry are created from the resulting diamonds. Synthetic stones are also used. Products made from them look beautiful, but there are differences. The presence of the smallest inclusions, the shade of the edges and the influence of the magnet will give away an artificial diamond.
Second-class materials are used in technical products. Whole crystals, fragments and even “dust” from grinding the mineral go to a just cause. Diamonds of the appropriate type and size are selected for bearings, drill tips, and drills. Raw crystals with a sharp tip are used in electronics. Small, defective specimens and fragments are crushed into diamond powder. The crumbs are sprayed onto the edges and planes of cutting and sharpening discs and grinding wheels.
Production of artificial graphite
Graphite and its properties are not the only thing you should know if you are interested in this mineral. It is also important to inquire about the production of an artificial variety. It differs from natural material in that during synthesis a substance with specified parameters is obtained.
Waste petroleum coke and coal sand are used for production. The mixture of fine-grained elements is fired and then cooled for about 5 weeks. The impact of temperature at the first stage is accompanied by its increase to 1200 °C.
To increase the theoretical density of graphite, the workpieces are impregnated with sand. At the final stage, graphitization occurs; it involves heat treatment of the material in a special furnace, where the temperature reaches 3000 °C. In this case, it is possible to form a crystal lattice.
This graphite has high thermal conductivity and excellent electrical conductivity. Anisotropy of properties is inherent in a mineral obtained by extrusion. Today, a newer technology is used, which is called isostatic pressing. This makes it possible to produce a material with a low coefficient of friction. It has isotropic properties.
The density of graphite (g/cm3), which is obtained during the extrusion process, reaches 2.23. The same indicator for the isostatic recrystallized variety, depending on the brand, can reach 5 g/cm3. This material is used for the production of large-sized workpieces, the length and diameter of which are 1000 and 500 mm, respectively, as well as for the production of casting parts and molds that have anti-friction properties.
Melting temperature
The range of temperatures at which graphite can melt is very diverse. Much depends, for example, on the final objectives of the operation. The temperature range is also determined by external conditions, the characteristics of the composition of a particular mineral, and the use of additional means of influencing graphite during heat treatment. The melting temperature at which it is possible to obtain graphite ready for use varies from 2600 to 3800 °C. Calculation on the Kelvin scale is also practiced. In this case, it already reaches 4000° K, but this value can also increase depending on the pressure indicator. Typically, graphite is melted under pressure of 105 – 130 Bar.
Graphite: melting point, properties and applications
Graphite is one of the minerals that is distinguished by its versatility in practical use. It is usually customary to associate it with coloring substances, but its capabilities are not limited to this. At the same time, we cannot talk about the universality of use of this material, since its layered structure also determines the scope of application. And this is not to mention the need to create special processing conditions. The fact is that the melting point of graphite in degrees Celsius can reach 2800 °C, which requires the use of special facilities for the manufacture of final products.
Application in the food industry
The presented substance is also widely used in the food industry. To do this, it undergoes certain processing during production. The densities of iron, ethyl alcohol, graphite and sugar are, for obvious reasons, different. But the presented material may both contain and be part of certain substances. It is found in paraffins, ethers, alcohol and even sugar.
You can verify this by conducting a simple experiment. First you need to take a piece of sugar. It is placed on a hard lid and covered with a cap (you can use a thimble). The metal covering the sugar is then heated to high temperatures. Over time, acrid smoke will begin to emerge from under the thimble. If you put a match near it, the gas will burn.
When the smoke stops coming out, you can remove the thimble. A black mass remains on the lid. This is coal. It represents the carbon from which graphite is made.
ELECTRICAL CONDUCTIVITY OF MINERAL
Graphite is an excellent conductor of electricity - in this indicator it is superior, for example, to mercury. Heating the mineral helps improve the conductivity of electrical current. Thus, the mineral has a negative temperature coefficient of resistance. At 0 degrees it is in the range of 0.39-0.602 ohms. As for the resistivity limit, it is the same for all types of material and is 0.0075 Ohm. These properties explain the widespread use of graphite in electrometallurgy.
Boiling temperature
The need for heat treatment is driven by the fact that enterprises seek to modify the performance properties of the material in order to create more efficient products. Methods of bringing the mineral to a boil are less commonly used, but they can also improve certain properties of the structure. The question of what is the melting point and boiling point of graphite often involves indicating the same range - from 3800 to 4200 ° C. The lower threshold determines the melting state, and the upper threshold determines the boiling state of the material. Again, depending on the characteristics of graphite and its variety, the conditions of thermal action in terms of obtaining the desired state of the mineral - boiling or melting - may converge.
Structure
When considering what density graphite has, as well as its properties and types, it is necessary to pay attention to its structure. This is a layered substance. Its carbon atoms are arranged in a crystal lattice, similar to a honeycomb. Hexagons in one layer fit tightly to each other. However, the connection between each level is weak. It is this feature that makes graphite easy to break.
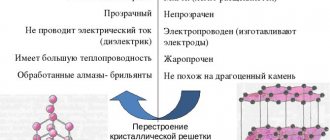
According to the Mohs scale, the hardness of the material is equal to one. For comparison, for diamond this indicator is 10, and for porcelain stoneware – 5. At a temperature of 1500°C, according to scientists’ research, the crystal lattice of graphite can be transformed into diamond.
During industrial processing, the structure of the substance changes. At the same time, different grades of graphite have different properties. If the extracted material has not been processed artificially, it is a natural type of substance.
Application of graphite
The properties of graphite, as already noted, have allowed it to find wide application in a variety of fields. It is used in the manufacture of electrodes, pencils, protective equipment, reference measurement materials and even as a lubricant. The thermal properties of the mineral determined its usefulness as part of furnace structures. For example, lining plates and melting crucibles are made from graphite. But here, too, much depends on the specific variety in which graphite is presented. The melting point of some types of material, which is 2600 °C, for example, does not allow their use in industrial heat treatment chambers. But the electrochemical qualities allow the use of most graphite products as elements of conductive infrastructure.
Examples of problem solving
| Did you like the site? Tell your friends! |
News & Events
"PKNM" was provided with preferential financing from one of the largest banks in the country
VTB Bank in Perm within the framework of the Program to stimulate lending to small and medium-sized businesses (“Program 1706”), implemented by the Ministry of Economic Development ...
The petrochemical cluster approved the composition of the Association's board
The next General Meeting of members of the Association for Assistance to the Development of the Petrochemical Industrial Cluster of the Omsk Region was held. The participants elected the Chairman of the General Meeting, his...
Research and production enterprise "POLIPLASTIC" enters the European market
On June 27, 2022, the first International Exhibition Compounding World Expo 2022 will begin its work in Essen (Germany). The event promises to be the largest event for suppliers of…
The testing laboratory of the Novocherkassk enterprise of the Titan Group of Companies has been replenished with new equipment
In June, the industrial testing laboratory of the Novocherkassk Lubricants Plant (part of the Group) was replenished with a new domestic Engler viscometer...
analyzed the results of polypropylene sales for the first five months
(a joint venture of Titan Group of Companies, Gazprom Neft and SIBUR) analyzed the results of polypropylene sales for the first five months of 2022. Sales volume in Russia...
Research and production enterprise "POLIPLASTIC" shared its experience in digitalization of production processes
Evgeniy Ambrosov, technical director of the Research and Production Enterprise “POLIPLASTIC”, took part in the round table “Chemical and petrochemical industry of Russia in the era...
Information
Cast iron pipes with nodular graphite
“PKNM” was provided with preferential financing from one of the largest banks in the country The petrochemical cluster approved the composition of the board of the Association The scientific and production enterprise “POLIPLASTIC” enters the European market
Atg graphite
“PKNM” was provided with preferential financing from one of the largest banks in the country The petrochemical cluster approved the composition of the board of the Association The scientific and production enterprise “POLIPLASTIC” enters the European market
Catalog of organizations and enterprises
Graphite-USSO Graphite products
Stalex
Special steels and materials, custom production, graphite...
Chelyabinsk Electrode Plant
Industry: Not known Criminal Code: 5508.96 thousand rubles. Address: 454038, Chelyabinsk Electrotechnical Plant, Chelyabinsk, Chelyabinsk region. Manufactured products: artificial graphite; polyacrylonitrile (carbon) harness; products made from artificial...
OOPS
CJSC UglerodPromServis is a large producer of graphite and carbon-containing materials in the Ural region. We produce graphite according to specified characteristics and requirements...
GraphitePro
Graphite. Graphite products.