Cryolite was known to the Eskimos long before its discovery by Europeans. The name of the mineral comes from the Greek “krios” - ice and lithos - stone (ice stone) (d'Andrada, 1800); first reliably studied by Abilgard (1799).
The English name of the mineral is Cryolite
Synonyms: Ice stone - Eisstein (Glocker, 1831), orsugisat - Orsugisat - local designation in Greenland, meaning “fat salt” (according to Hinze); corresponds to artificial α-cryolite.
Cryolite
Formula
Na3AlF6
Physical and chemical properties
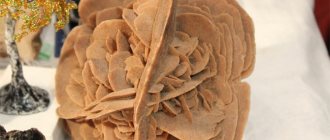
The fragile mineral, in addition to white and colorless shades, can have red-brown, black, smoky and black colors, while their shine varies from oily to pearlescent. Crystals appear in the form of lamellar and dense drusen, as well as coarse-grained solid masses. Depending on the origin, hydrothermal or endogenous, it is found in close proximity to quartz, amazonites, galenas, pyrites, fluorites, albites and siderites.
In the chemical composition of Na3[AlF6], the main elements are: 12.8% - aluminum, 32.8% - sodium, 54.4% - iron. Cryolite begins to melt at temperatures above 1000°C, giving the flame a red-yellow hue. Up to 0.037% of the stone dissolves in water; in acids it is completely broken down.
The density of the mineral is 2.97 g/cm³, the fracture is uneven, and the split is in two directions.
Form of being in nature
The appearance of crystals. Crystals are pseudotetragonal and pseudocubic in appearance; crystals formed by m(110) and c(001) sharply predominate (compose pseudocubes); the r(110), v(101) and k(101) faces are less developed, which sometimes reach significant development with the formation pseudocubooctahedra. Rarely observed crystals of the second generation are richer in shape and sometimes form crystals of unusual habit, flattened along (001). The m(110) faces often have shading parallel to the edges. There are parallel stepwise intergrowths of crystals.
Mineral twins. Doubles are very common; Granular cryolite is always polysynthetically twinned. There are at least 14 laws of twinning, a significant part of which is established only in granular cryolite and is explained, according to most authors, by the influence of mechanical stresses that arose during the cooling of high-temperature cubic β-cryolite and during its transformation into a monoclinic α-modification. Such twins were reproduced experimentally. Twins usually appear immediately in each grain according to several laws. The general geometric patterns of this phenomenon have been considered by many authors. Various twinning laws, manifesting themselves together, increase the overall symmetry of the intergrowth to rhombic, tetragonal or cubic. Diagnosis of various types of twins presents great methodological difficulties; The laws indicated by various researchers are not accepted by everyone. The following types of twins were observed (according to Dana, 1951):
- twin axis [110], rotation 90°. Twins of fusion and germination are common, sometimes quadruples. Twinning according to this law is very typical for coarse-grained minerals (the so-called “Baumhauer’s law”);
- twin axis [110], rotation 180°; repeat twins are observed in granular minerals, but are less common in individual crystals. Artificially obtained by cooling a heated stone;
- twin axis [021], rotation 120°; the fusion surface is irregular. Artificially obtained by cooling a heated mineral. In the form of thin plates they are common in granular cryolite (the so-called “new law” of Böggild);
- twin axis [111], rotation 180°; the rhombic section is close to (110). Doubles of repeated type. Rare, found only on crystals, unknown in granular cryolite;
- twin axis [100], rotation 180°, fusion plane (001); found only in granular cryolite, for which they are common;
- by (100), 180° rotation around [001], fusion plane (100); found only in granular cryolite; thin plates; common;
- twin plane and fusion plane (112); found only in granular mineral; records; common;
- twin plane and fusion plane (112); found only in granular mineral; records; common;
- twin plane and fusion plane (110); records; found only in coarse-grained cryolite from the Urals;
- twin axis [111], rotation 180°; the rhombic section is not a possible face, but is close to (110); were not established by Böggild, but it is possible that this is the “law d” of Kroes and Hillebrand; found only in granular cryolite;
- but (211); one of Padurov's new laws; apparently very rare;
- twin axis [001], rotation 90°; very close to 9;
- twin axis [201], rotation 120°; very close to 8;
- twin axis [201], rotation 120°; very close to 7.
For twin crystals, the 1st law is most common, less so the 2nd and 4th. Doubles according to other laws are found only in granular stone. There appear to be epitaxial intergrowths between high-temperature cubic p-cryolite and cryolithionite; the orientation of minerals in the intergrowths has not been established. Epitaxial intergrowths with thenardite were artificially obtained.
Aggregates . Granular discharge, individual grains, crystals, porcelain-like discharge mixed with opal.
Mineral deposits
There are not many places in nature rich in cryolite accumulations. It is mined on an industrial scale only in one place in the world - the mining settlement of Ivittuut, located in Western Greenland. New Zealand is famous for its massive deposits of high-quality cryolite. Single deposits of cryolite are located in Germany and the USA. Canada, Namibia, Nigeria and Ukraine.
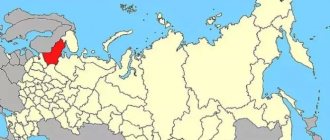
In Russia, compared to the demand indicator, the volumes of mined cryolite are extremely small. Accumulations of the mineral are found in the Murmansk region, in the Southern Urals in the vicinity of the Ilmen Mountains, as well as in the southwestern region of Primorsky Krai.
Chemical composition
Chemical theoretical composition: Na - 32.86; Al - 12.84; F - 54.30%. There are no chemical analyzes of cryolite that fully meet modern requirements; the limits of fluctuations in composition and the possibility of isomorphic occurrence of various elements in the crystal lattice of the mineral have not been established. Most analyzes detect a discrepancy between the amounts of cations and anions. More often a deficiency of F is detected, less often - its excess. The Na:Al ratio never corresponds to the theoretical one and in most analyzes ranges from 2.92 to 2.99 (0.36 - 0.04 wt.% Na below theoretical). These results are likely due to imperfect analysis methods. The discrepancies between the theoretical composition and analyzes of synthetic and natural minerals performed during various physicochemical and technological studies are especially large. Various variations of the cryolite formula have been proposed. Isomorphism is possible between high-temperature β-cryolite and isostructural compounds K3AlF6 and elpasolite (K2NaAlF6); in cryolite from the Ilmen Mountains, 0.08% K was discovered by flame photometry, and in cryolite from Tuva - 0.0028 Li2O, 0.0008 Rb2O and 0.0008 Cs2O, 0.07% K, 0.007 Li2O, 0.0003 Rb2O , Seine discovered. The often observed Ca is probably due to the presence of admixtures of other minerals, although Nöllner and Lemberg assumed an isomorphic replacement of sodium with calcium. Often observed in the mineral, Si and Mg are due to impurities of other minerals, and increased amounts of Li are due to the presence of cryolithionite.
Crystallographic characteristics
Syngony. Monoclinic. L2PC
Symmetry class . Prismatic - 2/m. Axis relationship. 0.973: 1: 1.391; р=90°11′.
Crystal structure
In the cryolite structure, discrete, slightly deformed AlF6 octahedra are located at the vertices and in the center of the cubic unit cell. Between them are Na atoms, 1/3 of which have coordination 6, and 2/3 - 12. NaF6 octahedra are located in the middle of the vertical edges and in the centers of the basal planes of the unit cell, and have common faces with cuboctahedra occupying the spaces between the AlF6 and NaF6 octahedra , in the centers of which the remaining 2/3 of the Na atoms are located. In another interpretation, the cryolite structure consists of chain NaAlF6 groups parallel to the c axis, with channels containing NaF groups.
Distances between atoms in AlF6 -Al octahedra - F = 1.79 - 1.83; in NaF6 octahedra Na - F = 2.42 - 2.32; for other Na atoms, the Na - F distance ranges from 2.21 to 2.68 A (Naray-Szabo and Sasvari).
High-temperature β-cryolite—cubic. The structure is of the (NH4)3AlF6 type, close to the structure of elpasolite. Upon cooling, due to the insufficient size of Na ions located in the centers of the cuboctahedra, the structure turns into a monoclinic one—the inclination of the polyhedra changes, and differences in the length of their edges appear. The (110) plane of monoclinic α-Na3AlF6 cryolite corresponds to the plane of a pseudocube, very close in size to the cell of elpasolite and (NH4)3AlF6. During the polymorphic transformation, several systems of polysynthetic twins are formed.
Artificial cryolite
The rarity of cryolite of natural origin and the widespread need for this mineral in industrial sectors forced representatives of domestic science to think about making an artificial analogue.
Russian scientists made their first attempts in 1924, and 9 years later the first production plant was opened in the vicinity of Sverdlovsk.
Synthetic cryolite is produced in several ways:
- using fluorite raw materials;
- by combining sodium fluorite and aluminum;
- under the influence of hydrofluoric acid on aluminum hydroxide present in soda.
Cryolite is produced in the form of a fine powder, the color of which varies from pale pink to gray-white. It is dangerous to human health, so protective equipment should be used when coming into contact with it. Separate requirements are imposed on its storage and transportation, for which special disposable containers are used - soft containers or special multi-layer bags. The material is resistant to fire and explosions, but can release toxins when exposed to high temperatures.
Scope and scope
Oddly enough, this mineral is in great demand in industry, and sometimes is simply irreplaceable. But due to the fact that this natural stone is very rare and is practically not mined in industrial quantities, the need arose to create its synthetic analogue. It was the Soviet Union that became the pioneer in this area and already in the first half of the 20th century, a plant for the production of artificial cryolite was opened in the Sverdlovsk region.
Zodiac sign
Cryolite is considered the stone of Aries, Virgo, Capricorn and Aquarius. It cools their ardor, controls their emotional mood, makes them be restrained and attentive. People born under these signs must have something with Cryolite with them. Moreover, the setting for the stone does not play any role. It has a weaker effect on Sagittarius and Leo, but they are still recommended to wear jewelry made from this mineral. Who this gem is definitely not suitable for is Pisces. It makes them completely infantile, lethargic, and depressed. For other zodiac signs it is simply neutral.
Medicinal properties
It has no special medicinal properties. But, undoubtedly, they exist. It has been noted that the presence of this stone in the room calms the nerves, reduces headaches, stabilizes the heartbeat, normalizes blood pressure, reduces muscle tone and improves mood. It also has a beneficial effect on the body's indications, has a positive effect on the functioning of the gastrointestinal tract, cures colds at an early stage of their development, strengthens the immune system, and helps get rid of negative dreams. Moreover, the finishing method does not affect the properties of the stone.
Magic properties
Cryolite does not have such a strong aura to work miracles. However, the absorbed earthly energy is enough for him to protect his owner from the evil eye and the negative influence of envious people. If you keep it in the house, it will avoid theft. No magical rituals are performed with it, but its owner can safely enlist the support of the stone in serious negotiations, especially if they concern the expenditure of significant funds. He will not allow dishonest people to fool their master.
Decorations
Despite its fragility, Cryolite is actively used to create jewelry. But not all specimens can be used, but only those that have a uniform color and significant size. There are many options on how to wear this stone. Rings, necklaces, necklaces, rings, pendants, pendants, bracelets and much more are made from it. Such beauty is also used in famous brands. Cryolite goes well with other stones.
Household use
It may not be strange, but Cryolite is used in various fields. For example, it is an integral component of the process of producing aluminum by electrolysis. Due to the shortage of this gem, which is mined naturally, it began to be artificially grown in large quantities. Melt cryolite is often used to produce glasses, enamels and acids. Moreover, the use of gems gives the glass greater durability and strength. Thanks to Cryolite, impact-resistant frosted glass was created. As for the enamel industry, this stone performs the function of a filler and the function of an abrasive.
To create artificial Cryolite, you must have a fluorite product. And its quantity on earth is simply off the charts, so there will be no shortage of raw materials. Hydrofluoric acids, certain types of reagents, pesticides and insecticides are produced from cryolite powder. Pesticides are an indispensable tool for protecting grapevines, fruit trees, vegetables and ornamental plants from the invasion of all kinds of pests. Fireworks are also made using Cryolite, which allows them to glow bright yellow.
There are several methods for producing synthetic Cryolite:
- using a compound of aluminum and sodium fluorite;
- using fluorite substance;
- through the influence of hydrofluoric acid on aluminum hydroxide, which is present in soda.
Artificial Cryolite has the appearance of a powder with a pale pink or gray-white tint. Its cost is moderate. For one bag weighing 25 kilograms you need to pay about 3,000 rubles. Direct contact of a person with it can have a detrimental effect on the condition of his body, therefore it is necessary to work with this stone exclusively in personal protective equipment.
There are certain requirements for its transportation and storage. For this purpose, disposable containers are used: either special-purpose multilayer bags or soft containers. Such a substance does not ignite well and practically does not explode when exposed to high temperatures, but if the combustion process does occur, then the vapors released are very toxic and must be feared.
Where is cryolite used?
By limiting the volume of natural cryolite in the world's deposits, it is effectively replaced by a synthetic analogue in industrial areas. The mineral is involved in the production of aluminum alloys by electrolysis of bauxite, in which aluminum oxide dissolves most effectively under the influence of molten cryolite.
In the glass industry, cryolite is used in the production of high-strength white glass, and in the chemical industry - in the production of enamel compounds and hydrofluoric acid.
Unique samples of natural cryolite are exhibited in the collections of jewelers and mineralogists.
Artificial cryolite is used in the production of pesticides, insecticides, reagents and fireworks.
Uses [edit]
Molten cryolite is used as a solvent for aluminum oxide (Al 2 O 3 ) in the Hall–Heroult process used in aluminum refining. This reduces the melting point of the molten (liquid state) aluminum oxide from 2000 to 2500 °C to 900–1000 °C and increases its conductivity [ citation needed
], making aluminum extraction more economical. [13]
Cryolite is used as an insecticide and pesticide. [14] It is also used to give fireworks their yellow color. [15]
Medicinal properties
The mineral has not been fully studied by lithotherapists , but it has been noted that constant contact with cryolite affects the psycho-emotional state and some vital systems.
The stone calms the nerves, improves mood, normalizes sleep, and relieves nightmares. It has a beneficial effect on the immune system and blood pressure, and is used for the prevention and treatment of colds at the initial stage. Its positive effects on the cardiac, digestive and muscular systems have also been noted.
Magic properties
Cryolite interspersed with siderite crystals
Cryolite is not used by esotericists in various rituals, but this does not mean that it does not have magical properties. The stone has the energy of the Earth and is a strong amulet against evil spirits, envy, troubles, dangers and fatal accidents. A talisman with a stone will protect its owner from rash decisions and financial losses.
In order for the properties of the stone to be revealed as much as possible, you must constantly keep it with you and communicate with it. It is recommended to carry a talisman or amulet in your pocket and periodically take it in your hand. If the amulet stone is in the house, then you should devote a little time to it every day and talk to it.
Stone care
Cryolite is a brittle mineral that is not resistant to external influences. In order for it to maintain its aesthetic and physical properties, you should adhere to the basic rules of caring for it:
- Under no circumstances should you store the stone with other minerals; for this you need to use a separate box with fabric upholstery;
- do not leave cryolite in direct sunlight;
- do not allow moisture, especially drops of dew and rain, to come into contact with the surface of the stone;
- remove the stone during physical activity;
- For cleaning, use a dry flannel cloth; do not use products containing abrasives or acidic compounds.
Aluminum refining
Aluminum produced by electrolysis is called raw aluminum. It contains metallic and non-metallic impurities and gases. Impurities are removed by refining by blowing the aluminum melt with chlorine. The resulting vaporous aluminum chloride, passing through the molten metal, envelops particles of impurities that float to the surface of the metal and are removed. Chlorination of aluminum helps remove gases dissolved in aluminum.
Then the liquid aluminum is kept in a ladle at a temperature of 700 - 730 ° C for non-metallic inclusions to float and gases to release from the metal. After refining, the purity of aluminum is 99.5 - 99.8%. For most consumers, aluminum of this purity is quite suitable. However, certain branches of modern technology require aluminum of higher purity. Such aluminum is obtained by electrolytic refining, in which contaminated aluminum serves as the anode and is dissolved and deposited on the cathode, and pure aluminum is the cathode. This refining produces aluminum with a purity of 99.996%.
If it is necessary to obtain aluminum of higher purity, the method of zone melting or distillation of aluminum is used.
In zone melting, rods are cast from electrolytically refined aluminum and placed in a quartz tube, in which a vacuum is created. An inductor is placed around the tube, connected to a source of high-frequency electric current (HF). Under the inductor, the rod melts and a zone of liquid aluminum appears, while the rest of the rod remains solid. The inductor moves along the rod at a certain speed and the zone of liquid aluminum moves. In this case, impurities are concentrated in the melt and move with it to the end of the ingot. The ingot is then removed and the end is cut off. The remainder consists of high purity aluminum (99.9999%).
When using the aluminum distillation method, its refining is carried out through the so-called sub-compounds by passing vaporous aluminum chloride and fluoride over molten aluminum at a temperature of 1000 °C and above.
These subcompounds decompose when cooled into aluminum and aluminum chloride or aluminum fluoride. The impurities contained in crude aluminum are not distilled. This method produces aluminum of very high purity (99.99999%).
The last two refining methods are expensive and inefficient. They are used to purify only small amounts of metal needed to make semiconductors and other critical products.
Read more: Aluminum refining
Zodiac sign
Cryolite has been little studied in astrology. Representatives of the zodiac system with a hot-tempered and explosive character should choose it as a talisman.
It is recommended for Capricorns, Aquarius, Aries and Virgos to have cryolite with them at all times. Their gem will teach them to control their emotions and make them more restrained towards themselves and others.
The influence of the stone on Leo and Sagittarius is not so pronounced. The mineral is not suitable for Pisces, as it increases their tendency towards immaturity and depressive mood.
The stone is neutral towards other signs of the Zodiac. When choosing cryolite, they are advised to rely solely on their inner instinct and sympathy.
Story
The origin of the name of the stone is as follows. At the end of the 18th century, a fairly large fragment of stone, very similar to a block of ice, was found in Greenland. The naturalist scientist from Denmark, Peter Christian Abildgaard, became interested in him. It was he who completely examined it and gave a comprehensive description. The beauty of the find was so amazing and impressive that the naturalist gave it the name “Ice Stone” or “Frost Stone” from two Greek words “krios”, which means “ice” and “lithos” - “stone”. The year of its opening is considered to be 1799.