Natural stone is a hard type of rock of natural origin or a mineral of the same type, excluding metals and sand. This stone is one of the oldest materials used by mankind for the construction of bridges, houses and facade cladding. Thanks to its shapes and natural beauty, this material will be an excellent decoration for any home, temple, palace or estate.
In more modern construction, natural stone is most often used as a facing material, both for external and internal cladding. In this industry, it has found its popularity due to the large selection of colors, patterns and designs, the combination of which can be selected for almost every type of home or premises.
The main properties of natural stone include: excellent wear resistance, high resistance to frost and moisture.
What is this characteristic?
For any substance, density refers to mass divided by unit volume. Since rocks (i.e., minerals) have a heterogeneous composition and include elements of different atomic masses, the physical characteristics of their density can vary significantly. Also, the density of stones depends not only on the gravity of the elements that make them up, but also on how tightly the elementary particles are “packed” in their internal structure.
Mineralogy deals with the study of mineral density. The density of a stone is calculated by dividing the mass of a mineral sample per unit volume by the mass of water of the same volume at a temperature of 4 ⁰C. For example, the sample weight is 200 grams. Water in the same volume 40 grams. In this case, the density of this stone will be equal to 5.
The density of stones is measured in kilograms per cubic meter or grams per cubic centimeter.
Specific Gravity Calculations
To carry out correct calculations, it is necessary, first, to understand what the concept itself means. So, specific gravity is the ratio of the weight of the proposed material to its occupied volume. All calculations are carried out according to the following formula: y=p*g, where y is specific gravity, p is density, g is the acceleration of gravity, which in normal cases is a constant and equals 9.81 m/s*s. The result obtained is measured in Newtons divided by cubic meter (N/m3). For subsequent conversion to the SI system (kg/m3), this result must be multiplied by 0.102.
The density of the proposed material is its mass in kilograms, which fits in a cubic meter. This parameter is very ambiguous and depends on many factors. For example, such as temperature. So, the average density of natural stone ranges from 1100 to 2700 kg/m3. Such a large range is due to the presence of a large number of types of natural stone, which, in turn, also affects such a parameter as the weight of a cube of natural stone.
How to find the density of a stone?
How is the density of a stone determined? The procedure is quite simple - we weigh the sample first in air, then in water. According to Archimedes' law, the resulting difference corresponds to the mass of water that the sample displaces. Density is calculated by dividing the mass of the sample in air by this difference.
Depending on their density, minerals can be light, medium, heavy or very heavy. For example, the density of granite stone is 2,600 kg/m³. For reference: the density of light does not exceed 2.5 g/cm³, medium - ranges from 2.5 to 4 g/cm³, heavy - from 4 to 8 g/cm³. Minerals with a density above 8 g/cm³ are considered very heavy stones.
Density of natural stone
Density is the mass of a unit volume of a substance. The weight of the structure depends on this indicator: the higher the density of the stone, the heavier the structure will be. Based on density, stones are divided into light ones (density up to 2200 kg/m3): dolomites, limestones, travertine and shell rock. And heavy (density more than 2200 kg/m3): quartzites, shales, granites. Density depends on the porosity of the rock and the minerals included in its composition. Water absorption and, accordingly, salt and acid resistance depend on porosity. And these are the main indicators that affect the durability of the material. In addition, the total porosity determines the strength, thermal conductivity, polishability, workability, decorativeness of the stone and other quality characteristics.
Gem Density
In addition to density and another characteristic - hardness, semi-precious minerals or precious stones also have such an important component as mass, measured in grams or carats (for pearls - in grains).
To understand the relationship between these units, remember: 1 carat corresponds to 200 milligrams, one grain equals 50 milligrams, that is, 1 carat is equal to four grains. The accuracy of measuring gems is up to two decimal places.
Specific Gravity of Gemstones (Density Table)
Gemstone specific gravity is a designation for the relative density of a piece of jewelry or gemstone.
Every jeweler knows from experience that some stones are “by weight” heavier than others. For example, colorless zircon weighs more than a diamond of the same size, and sapphire weighs more than an emerald. Scientists have long learned to express this quality quantitatively, and this plays a big role in recognizing substances. Water was used as a standard, and the weight of each substance was compared with the weight of an equal volume of pure water. The number obtained as a result of this comparison is called the specific gravity, or relative density of the substance. Thus, the specific gravity of a body is the ratio of its weight to the weight of pure water of equal volume. To obtain accurate data, water at a temperature of 4°C is used as a standard.
Let's go to the laboratory
How to measure drag density. stones in laboratory conditions? The hydrostatic method is best suited for this. Its principle was proposed by the Greek scientist Archimedes many centuries ago. The essence of the principle, known from a school physics course, is this: a body immersed in a liquid is pushed out of it by a force that is equal to the weight of the liquid displaced by this body.
To put it simply, if you hang a stone and lower it into water, its weight will decrease compared to the original by as much as the volume of water displaced by it weighs. It is clear that this volume will be equal to the stone’s own volume.
Thus, by sequentially weighing stones in the air and then in water, we can obtain all the data we need for the calculation.
The hardest stone in the world
To assess the strength of minerals, a 10-point Mohs table and an absolute scale of linear hardness are used. In both grading systems, diamond was indicated as the hardest stone. But a natural gem without internal defects was taken as the standard.
In the 20th century, gemologists supplemented the list of Mohs reference samples with types of gems with intermediate hardness values. Now they are considering 20 degrees of strength: from 1 to 10 plus positions 1.5–9.5 (0.5 points added to the whole number).
Among jewelry, the first place is occupied by a diamond - the same natural diamond, only already cut. This is the hardest stone. Jewelers use specimens without hidden cracks or impurities, and a properly executed cut imparts strength.
Recent research has confirmed that there are harder gems on earth. Artificially synthesized diamond is stronger than its natural counterpart. Among the rocks of natural origin, lonsdaleite and wurtzite-like boron nitride are called the most durable. In the lists of polymer crystals, fullerite took first place.
Watch a program about how a diamond is created:
The most durable natural gem
Diamond is the hardest of all natural minerals known at the time of the creation of the Mohs scale. The name translated from Greek means “indestructible”.
Samples of the following colors are found in nature:
- black;
- colorless;
- yellow;
- red;
- blue;
- brown;
- green.
A colorless diamond is considered the strongest (due to the absence of impurities). Friedrich Mohs called it the standard of hardness and gave it 10 points. On the absolute scale of linear strength, the value is 1500. The gem easily scratches materials such as tempered glass and damages the surface of sapphire, ruby and other less durable rocks.
Diamond characteristics:
- chemical formula - C (consists of carbon, there is an admixture of nitrogen - N);
- when kept in a vacuum for a long time, it degenerates into graphite;
- color does not change when lighting changes;
- the fracture surface is uneven and splintered;
- physical properties - transparent, with a diamond shine;
- luminescence - blue, green, red and yellow reflections appear under solar or X-ray rays.
Samples unsuitable for jewelry are used in the manufacture of tips, discs, cutters, and other diamond-coated tools. They process softer natural and artificial materials.
See facts about the diamond stone:
The strongest synthetic stone
In places where meteorites fall with graphite, crystals called hexagonal diamonds are found. They are 50–58% harder than diamond.
Due to the tiny amount of natural hexagonal diamonds, the stones began to be synthesized. The artificially grown gem was named lonsdaleite, named after the crystallographer Kathleen Lonsdale.
Characteristics of lonsdaleite:
- the carbon lattice consists of 6 atoms;
- collapses under pressure of 150 GPa;
- obtained by compressing graphite at high temperatures.
Lonsdaleites are not used in decoration or industry due to the high financial costs of cultivation. Scientists are working to make synthesis cheaper.
To watch an overview video about the substance:
Everything - to nature!
Let us now turn to natural stone materials. As is known, there are several types of them. From a practical point of view, any rock is usually classified into one of two groups - strong or low-strength.
Materials of the first group have a high hardness index and, most often, a medium- or coarse-grained structure. In the so-called unweathered state, they have little water absorption. Other (low-strength) rocks, as the name implies, have much lower strength. They also have a much higher degree of water absorption.
Sometimes when recognizing types of stone, it is necessary to determine its hardness. In field conditions, it is most convenient to do this using the so-called. relative Mohs scale and additional available tools. Such improvised means can be a stylus, a coin, a piece of glass, a file, a steel needle or knife, an ordinary or diamond glass cutter. The average density of a stone is also important in determining its type. Having determined this value, you can identify the breed by referring to special tables.
The hardest materials on Earth
The most durable material in the world, which is harder than diamond, is polymerized fullerite. This material can easily scratch a diamond, as easily as if it were not a precious diamond, but ordinary plastic. This material is a structured crystal, the nodes of which consist of whole molecules rather than small atoms.
Lonsdaleite is also considered a strong material. This is a modification of allotropic carbon, which is close in hardness to diamond. This material was extracted from a meteorite crater. The origin of the material is graphite.
The third position in the hardness ranking is firmly occupied by wurtzite boron nitrite. The crystalline structure provides a high degree of strength to this material.
Nanostructured cubonite, or kingsongite. The unique capabilities of this material have ensured its frequent use in industry.
Carbon-boron nitrite occupies an honorable fifth position in our ranking. The main components of this material are boron atoms, as well as carbon and nitrogen.
Calculating the density of natural stones
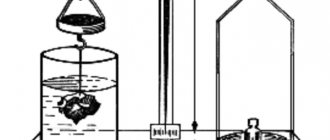
How to calculate the average density of a sample stone? The equipment required for this is a scale with a set of weights and the ability to measure the volume of an irregularly shaped sample.
The easiest way to do this is to have a graduated cylinder with a volume of about half a liter. 200-300 ml of water is poured into such a cylinder and a piece of the stone material being studied is placed.
The total volume of samples placed in water is determined by the amount of water displaced by them. Then, by dividing their mass by the calculated volume, the average density of the material is obtained.
Hydrostatic weighing method
This method is time consuming and comparatively cheaper.
Hydrostatic weighing method
First, a stone is hung on a thin wire from a hydrostatic balance and its weight is measured in air. Then the stone is immersed in water and its weight is measured again. After weighing, the water volume of the stone is determined by subtracting the weight of the stone in water from the weight of the stone in air. After determining the water volume of the stone, the density of the stone is calculated using the following formula P=M:V
- P is the density of the stone.
- M is the mass of the stone.
- V is the water volume of the stone which is determined by subtraction. V= suspended mass in air - suspended mass in water.
What is important to consider?
It should be noted that this method is only suitable for dense rocks with insignificant water absorption (no more than 2%). If this characteristic is higher (up to 5%), the dry sample, after having been weighed, must first be placed in an aqueous medium for saturation. The average density is then determined using the above method. Saturation is considered complete if weight stops increasing during water absorption.
Porous stones (most often limestone or tuff) have low strength. They are easy to process - use a regular hacksaw to cut out a sample of the desired shape (for example, a cube) and calculate its volume by measuring the edges.
Method of immersion in heavy liquids
This method is very complicated and much more expensive than the first, but it does not take much time. This method is used to identify real stones from artificial stones and fakes. This method is based on the property of heavy water in which solid objects do not sink to the bottom and do not float, but are as if suspended, provided that their density is the same. The stone is first placed in very heavy water, and the stone will be squeezed out to the surface by the water. Then the heavy water begins to be diluted with distilled water, while the density of the water will gradually decrease and when the density of the water becomes equal to the density of the stone, the stone will go into a suspended state. After this, you need to measure the density of the water and you can identify the stone using the table. The density of dilute heavy water is determined in laboratory conditions using special Westphal balances. Stone density table.
| Stone | Density | Stone | Density | Stone | Density |
| Tantalite | 5,18—8,20 | Diamond | 3,47—3,55 | Aquamarine | 2,67—2,71 |
| Cassiterite | 6,8—7,1 | Titanite | 3,52—3,54 | Eye of the Tiger | 2,64—2,71 |
| Wulfenite | 6,7—7,0 | Gemimorphite | 3,52—3,54 | Augelite | 2,7 |
| Galliant | 7,05 | G hypersten | 3,4—3,5 | Marble onyx | 2,7 |
| Cerussite | 6,46—6,57 | Singalit | 3,47—3,49 | Labradorite | 2,69—2,7 |
| Cuprite | 5,85—6,15 | Vesuvian | 3,32—3,42 | Corals | 2,6—2,7 |
| Phosgenite | 6,13 | Dumortierite | 3,26—3,41 | Vivianite | 2,6—2,7 |
| Crocoite | 5,9—6,1 | Epidote | 3,4 | Cordierite | 2,58—2,66 |
| Sheelit | 5,1—6,1 | Rodicite | 3,4 | Aventurine | 2,65 |
| Dzhevalit | 5,60—5,71 | Purpurite | 3,2—3,4 | Rock crystal | 2,65 |
| Zincite | 5,66 | Peridot (peridot) | 3.27—3.37 | Citrine | 2,65 |
| Proust | 5,57—5,64 | Jade | 3,30—3,36 | Prasiolite | 2,65 |
| Pyrite | 5,0—5,2 | Tanzanite | 3,35 | Smoky quartz (rauchtopaz) | 2,65 |
| Hematite | 4,95—5,16 | Dioptase | 3,28—3,35 | Rose quartz | 2,65 |
| Fabulite | 5,13 | Cornerupin | 3,28—3,35 | Amethyst | 2.63—2,65 |
| Chromite | 4,1—4,9 | Diopside | 3,27—3,31 | Aventurine feldspar | 2,62—2,65 |
| Ilmenite | 4,72 | Axinite | 3,27—3,29 | Agate | 2,60—2,65 |
| Zircon | 3,90—4,71 | Ekanite | 3,28 | Moss agate | 2,58—2,62 |
| YAG grenade | 4,6 | Enstatite | 3,26—3,28 | Eleolith | 2,55—2,65 |
| Barite | 4,5 | Tourmaline | 3,02—3,26 | Chalcedony | 2,58—2,64 |
| Smithsonite | 4,3—4,5 | Sillimanite | 3,25 | Chrysoprase | 2,58—2,64 |
| Psilomelan | 4,35 | Smaragdite | 3,25 | Peristerite | 2,61—2,63 |
| Witherite | 4,27—4,35 | Apatite | 3,17—3,23 | Moon rock | 2,56—2,62 |
| Rutile | 4,20—4,30 | Giddenite | 3,16—3,20 | Orthoclase | 2,56—2,60 |
| Chalcopyrite | 4,1—4,3 | Kunzite | 3,16—3,20 | Pseudofit | 2,5—2,6 |
| Spessartine | 4,12—4,20 | Lazulite | 3,1—3,2 | Variscite | 2,4—2,6 |
| Almandine | 3,95—4,20 | Fluorite | 3,18 | Obsidian | 2,3—2,6 |
| Rhinestone | 3,15—4,20 | Andalusite | 3,12—3,18 | G ovlite | 2,53—2,59 |
| Willemite | 3,89—4.18 | Magnesite | 3.00—3.12 | Sanidin | 2,57—2,58 |
| Painite | 4,1 | Euclase | 3,10 | Amazonite | 2,56—2,58 |
| Sphalerite | 4,08—4,10 | Tremolite | 2,9—3,1 | Thugtupit | 2,36—2,57 |
| Ruby | 3,97—4,05 | Actinolite | 3,03—3,07 | Leucite | 2,45—2,50 |
| Sapphire | 3,99—4,00 | Amblygonitis | 3,01—3,03 | Cancrinitis | 2.4—2,5 |
| Celestine | 3,97—4,05 | Nephritis | 2,90—3,02 | Apophyllite | 2,30—2,50 |
| Ganit | 3,99—4,00 | Danburite | 3,0 | Colemanite | 2,42 |
| Anataz | 3,58—3,98 | Datolite | 2,90—3,00 | Gayuin | 2,4 |
| Malachite | 3,82—3,95 | Brazilianite | 2,98—2,99 | Petalite | 2,40 |
| Azurite | 3,75—3,95 | Anhydrite | 2,90—2,99 | Thomsonite | 2.3—2,4 |
| Periclase | 3,7—3,9 | Phenakite | 2,95—2,97 | Chrysocolla | 2,00—2,40 |
| Pleonastus | 3,7—3,9 | Dolomite | 2,85—2,95 | Moldavite | 2,32—2,38 |
| Siderite | 3,85 | Aragonite | 2,94 | Hambergite | 2,35 |
| Demantoid | 3,82—3,85 | Prehnite | 2,87—2,93 | Alabaster (gypsum) | 2,30—2,33 |
| Staurolite | 3,7—3,8 | Jasper | 2,58—2,91 | Sodalite | 2,13—2,29 |
| Pyrope | 3,65—3,80 | Lapis lazuli | 2,4—2,9 | Natrolite | 2,20—2,25 |
| Uvarovite | 3,77 | Beryllonite | 2,80—2,85 | Stichtitis | about 2.2 |
| Alexandrite | 3,70—3,73 | Ward it | 2,81 | Opal | 1,98—2,20 |
| Chrysoberyl | 3,70—3,72 | Soapstone (wen) | 2,7—2,8 | Sulfur | 2,05—2,08 |
| Rhodonite | 3,40—3,70 | Turquoise | 2.60—2,80 | Meerschaum (sepiolite) | 2,0 |
| Rhodochrosite | 3,30—3,70 | Serpentine | 2,4—2,8 | Ulexit | 1,9—2,0 |
| Kyanite | 3,65—3,69 | Garnierite | 2,3—2,8 | Ivory | 1,7—2,0 |
| Benitoite | 3,65—3,68 | Emerald | 2,67—2,78 | Geylussite | 1,99 |
| Rossular | 3,60—3,68 | Pearl | 2,60—2,78 | Kurnakovit | 1,86 |
| Baritocalcite | 3,66 | Beryl | 2,65—2,78 | Jet | 1,30—1,35 |
| Spinel | 3,58—3,61 | Bytovnit | 2,71—2,74 | Amber | 1,05—1,30 |
| Taafeit | 3,6 | Scapolite | 2,57—2,74 | ||
| Topaz | 3,53—3,56 | Calcite | 2,71 |
For this method, only heavy water is suitable and can be diluted with distilled water. Very often solutions of Thule, Clerici and Sushin are used as heavy water.
- Thule solution has a density of 3.2 and can identify a very large number of stones. This solution consists of double potassium iodide and mercury.
- Clerici's solution is toxic and has a density of 4.2, so it is used to identify heavier stones. This solution contains thallium formate and malonate, so this solution is very expensive.
- Sushin's solution has a density of 3.5. This solution consists of a solution of barium iodide and mercury.
All heavy solutions after dilution can be restored to their original density by conventional evaporation in a water bath. If the stone is clean and not diluted with other minerals, then you will determine the very accurate density of this stone using this method.
Source
Do it yourself
If there is no measuring cylinder of sufficient volume in the field, the amount of displaced water can be determined as follows. In any cylindrical metal vessel, just below the top, a hole is punched in the wall with an ordinary nail, then a tube is inserted into it, which you can also make yourself by rolling up any film. Fix it in the cylinder wall with plasticine or any similar material.
Thus, a traveling volume meter is obtained. If this unit is used constantly, it makes sense to solder the tube into steel or brass.
Man made stones
Everything written above applies to natural stones. And now it's time to talk about artificial ones. They can be wall, road and side. This should also include concrete roofing tiles and paving slabs, as well as all kinds of blind areas, stair steps and chimney elements.
In the production of almost all of the listed stones, both in Russia and abroad, strict technical standards are used. They regulate all the main characteristics - the quality of the starting materials, the dimensions and shape of the section, physical and mechanical parameters (including the density of concrete stones).
These requirements depend on the expected operating conditions and the available material.
Natural Stone Specific Gravity Table
Below is a table with all the necessary indicators, including the specific gravity of natural stone, which will help simplify all the necessary calculations.
Specific gravity and weight of 1 m3 of natural stone depending on units of measurement
| Material | Specific gravity (g/cm3) | Mohs hardness | Weight 1 m3 (kg) |
| Quartz Group | 2,6 | 7 | 2650 |
| Aluminosilicates group | From 2.5 to 2.76 | 6 | From 2550 to 2760 |
| Group of Ferrous-magnesian silicates | From 3.2 to 3.6 | 7 | From 3200 to 3600 |
| Carbonate Group | 2,7 | 3 | 2700 |
| Sulfate Group | 2,3 | 2 | 2300 |
Using this table, you can easily calculate such a parameter as the weight of natural stone.
What types of artificial stones can there be?
The concrete from which stones are made can be heavy or light. Artificial stones made from it are made solid or hollow. The standard characteristic of the average bulk density for hollow stones should not exceed 1,650 kg/m³, for solid stones - 2,200 kg/m³.
Wall stones in terms of average density (and, in addition, thermal conductivity) are considered effective (density up to 1,400 kg/m³), conditionally effective (1,400-1,650 kg/m³) and heavy (above 1,650 kg/m³). Most of them are now produced from lightweight concrete of low density (up to 1,800 kg/m³).
Heavy concrete (including sand) with high abrasion and low water absorption is used in the production of side or road stones, as well as paving slabs, since their operating conditions are more severe than those of wall slabs.
Artificial stones also differ in their aggregate, which can be quartz sand (considered a fine aggregate) or durable rocks (coarse aggregate). For example, the density of crushed stone made from natural stone can be different depending on the fraction - the degree of grinding. The composition of the aggregate also greatly affects the density of the artificial stone.
Porosity and water absorption of stone
With an increase in overall porosity, the strength and volume of the stone decreases, its polishability deteriorates, but the weight of the product decreases and its ability to be processed improves. According to the value of total porosity (P), natural stones can be divided into stones with low (P < 5%), medium (5% > P > 20%), high (20% < P < 40%) porosity. Another important property of rocks associated with porosity is the water absorption rate. The acid and salt resistance of the stone, as well as its frost resistance, depend on it and on the mineral composition of the material. With high water absorption and low porosity, cracks form in the material under this pressure. With high porosity of the stone, the crystallization pressure is distributed evenly and new cracks do not form (a striking example is limestone).