Gold is a metal familiar to everyone. Jewelry made from precious metal is popular, especially among women. Gold is one of the most expensive metals in the world and is one of the top three in terms of cost per gram. You can see different numbers on the products. They mean the sample of the metal. Having certain knowledge, you can determine the density of gold.
How to independently determine the specific gravity of gold + table of sample weights
Good afternoon.
Today I will continue talking about precious metals. We admire their shine in openwork items, price them in jewelry stores, weigh them in our hands, feeling the pleasant heaviness of the precious metal. Today my friend brought a ring and asks me to determine the material of the product. You can't tell by color whether it's gold or platinum. The sample is half erased and cannot be seen. From my school physics course, I vaguely remember that the specific gravity of gold is very high. Does this make any difference? I'll try to figure it out.
Drop some iodine
Rub the product on jeans or other thick fabric, then drop iodine on this place on the metal. High standard gold does not darken. If the metal has darkened (this may appear within a minute), the product is not genuine or the standard is not too high.
But brass and copper do not change color from iodine either. So this testing method is good for high-grade gold, but it is not enough on its own.
We do not recommend testing products with iodine levels of 585 or lower. After iodine, you will definitely have to polish them or remove stains with ammonia; traces may remain.
What is specific gravity
A conventional value showing how much a cube with sides of 1 meter (centimeter) will weigh is called specific gravity (volume weight). It is measured in kilograms per cubic meter (grams per cubic centimeter). At the Earth's poles, the force of gravity is greater, so the density of the substance (that is, the ratio of the mass of the metal to the volume) is taken as the basis for measurements, which differs by the value of the acceleration of gravity, depending on the location of measurement (from 9.780 m/s2 at the equator to 9.832 m/s2 at poles).
Specific gravity is confused with density; their numerical values in different measurement systems are the same. In my case, when the difference in the acceleration of free fall can be neglected, such a loose comparison is acceptable.
Table of specific gravity of gold depending on the sample
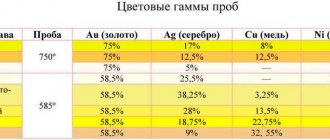
Gold is a soft metal. To improve its technological properties, various alloys are added to the alloy. The sample indicates the proportion of precious metal in thousandths of the alloy. Hallmark 999 is an alloy containing 999 parts of pure gold and 1 part of impurities. Impurities significantly affect the density of the alloy:
precious metal
The difference in density for the same alloy sample is due to the different composition of impurities (silver, palladium and copper, which paint products in different colors).
Draw with lapis pencil
A lapis pencil is needed to stop the bleeding. This is an antiseptic based on silver nitrate, it can be bought at a pharmacy, it costs about 100-150 rubles.
To check the authenticity of gold, soak the item in water. Then, using a lapis pencil, draw a small line over the wet one and wipe it off.
Lapis does not react with gold, but does react with other metals. If a trace remains, then the gold is fake or of very low standard. Nothing will happen to genuine metal.
Specific gravity and special properties of gold
Gold is not the only yellow mineral found in nature. Few people know how to tell by eye that a piece of jewelry is made from a gold alloy and that it is not a fake. The physical characteristics of gold and its main alloys are also useful in such a matter as refining gold from radio components. In order not to confuse it with other metals of a similar color, you need to know the density and specific gravity of gold.
Test with vinegar
Tell the seller that you will test the gold with vinegar. Are you nervous? There’s no need to run to the grocery store anymore. But a professional who is confident in his product will not sweat it.
Fake gold quickly darkens in vinegar - just pour a little into a glass and hold the metal in it for about five minutes. But real high-grade metal doesn’t care about anything.
physical characteristics
Gold is a fairly heavy metal; its density ranks seventh among all metals on the periodic table and is 19.32 g per cubic centimeter. Specific gravity is expressed differently in different unit systems. In the SI system it is newton per meter cubed, but most often jewelers use a non-systemic unit - grams per centimeter cubed. This is done for convenience, so the density will be equal to the specific gravity.
Gold has the following physical properties:
The peculiarity of gold is its inertness. It is this characteristic that allows the metal to have the status of noble. This makes it possible to produce gold jewelry, because it does not oxidize and retains its original appearance for a long time. For the jewelry industry, there is only one disadvantage of gold - its softness. This drawback is circumvented by adding other metals to the alloy - alloying, which changes not only this characteristic, but also others: density, melting point.
Studying the quality of a product using special means
Some properties of different grades of gold are presented in the table.
| Density g/cm3 | ||
| 375 | 37,5 | 11,55 |
| 585 | 58,5 | 12,85–13,24 |
| 750 | 75,0 | 15,3–16,6 |
| 999 | 99,9 | 19,3 |
| Try | Brand | Color | Compound | Density g/cm3 | T melt | |
| Silver (+- 0.5%) | Palladium | Nickel | Zinc | |||
| 375 | ZlSrM 375-160 | red | 16 | 11.54 | 880-900 | |
| ZlSrPdM | cream | 10 | 3,5-4,1 | 11.56 | 860-975 | |
| 375-100-38 | ||||||
| 585 | ZlNTsM 585-125-60 | white | 12,0-13,0 | 3,6-4,4 | 12.85 | 870-950 |
| ZlSrPd 585-225-160 | white | 25.5 | Ost. | 14.76 | 1175-1220 | |
| ZlSrM 585-300 | green | 30 | 13.92 | 835-880 | ||
| ZlSrM 585-200 | yellow | 20 | 13.6 | 830-845 | ||
| ZlSrM 585-80 | red | 8 | 13.24 | 880-905 | ||
| 750 | ZlSrNTs 750-125 | white | 12.5 | 15.38 | 900-950 | |
| ZlSrPd 750-100-150 | 15 | Ost. | 16.44 | 1250-1300 | ||
| ZlSr 750-250 | green | 25 | 15.96 | 1040-1045 | ||
| ZlSrM 750-150 | yellow | 15 | 15.45 | 885-900 |
High electrical conductivity characteristics along with inertness have made gold also an industrial metal. In terms of distribution, it is inferior to silver, but surpasses it in another quality: over time, silver oxidizes, but gold does not.
Specific gravity also plays a role in gold mining and in the production of alloys from it. High-quality alloys are obtained only by fusing metals with a small difference in density. Metals with a greater weight than gold can float during melting and form an inhomogeneous structure.
The melting point of gold is 1095 degrees. In addition, there is a hidden melting point. It is measured in kilocalories and represents the energy required to destroy the crystalline structure after the metal has been heated to its melting point. Gold is a diamagnetic metal - a metal that is not attracted to a magnet.
In the mid-twentieth century, the United States decided to store the country's gold reserves only in bullion. The weight of the bar was set at 400 troy ounces. Now the mass of a gold bar is 11–13.3 kg. One ounce is 31 grams.
Back in the Middle Ages, the weight of one gold coin was 30 grams, and it turned out that this weight measurement was very convenient for jewelers. It is still in use today. In addition, in the metal stock market, the price of gold is set in dollars per ounce, not per gram. So the mass of gold in the coin is one ounce.
How to determine specific gravity at home?
To do this, you will need scales whose accuracy is about a gram. After weighing into a glass or any other container where there are marks on the volume of liquid, lower the product and see how much the volume has changed. The mass divided by this indicator will be equal to the density of the metal, and accordingly, the specific gravity in units of g/cm?
Metals with similar specific gravity values
There are several metals in nature that have a density similar to gold. These are uranium and tungsten. Uranium will not be used to counterfeit gold for two reasons: firstly, it is radioactive, and secondly, it is not so easy to get. Tungsten is much more suitable for this. But tungsten also differs from gold in hardness and color, which does not allow it to be passed off as gold.
Counterfeiters do things differently. Tungsten ingots are coated with gold on top. But it's not just fake bars that are made this way. It is also used to make gold-plated jewelry. Externally, such products look the same as the real ones, but have different wear resistance characteristics and, accordingly, price. There are also simply tungsten products; they are popular among young people.
Jewelry made from lead, which has a soft structure, is also coated with gold. There is an opinion that lead can be easily distinguished by density or specific gravity, since this indicator is very different for gold. But that's not true. Gold is not used to make jewelry in its pure form, only in alloys.
In different alloys, the density of the product can vary, so products made of lead and 375 gold of the same size can weigh the same. Gold of other samples has a much higher density, with the exception of the rarely used 333 and the popular 417 in the West. The common 585 sample has a specific gravity of 15-16 g per centimeter cubed, which is significantly heavier than lead.
The density of gold is a very important indicator that is important in its mining, jewelry production, and refining. Determining the density can help distinguish a real product from a fake one, of course, if there is no sample on it, however, the sample can also be faked. This indicator is also important in research aimed at discovering new colors of metal.
Very often you can find jewelry with unusual colors; often these are sprayed, but alloys that have a rare color are especially valued. The production of such alloys is complicated precisely by the difference in melting temperatures and densities of the metals that must be added to gold to obtain a certain color.
Source
Test with black bread
The method is safe (for real gold!), but slow. Take the crumb of black bread, remember, mix with water and stick the mixture onto the product. Leave it until the bread crumb turns into a dry crust, then break it apart.
If there are dark traces inside the crumb, it means the fake has oxidized. If not, then it is either gold or good gilding.
Density of gold or specific gravity of gold
Understanding what the density of gold is, m3 or cm3, will help determine the standard of a gold product. High-grade gold 1 m3 weighs 19,300 kg, and 1 cm3 of high-grade gold weighs 19.3 g. Typically, the density of gold m3 (cube 1 x 1 x 1 m) is expressed in kg or tons per volume. To measure large volumes and mass of yellow precious metal, the density of gold in kg/m3 is most often used in calculations. These can be large bank gold bars in large quantities. If the weight of the precious metal is small, for example, like that of ordinary gold jewelry - a few grams, then in calculations it is customary to use the density of gold in g/cm3, since it is more suitable for this.
Don't try it on your teeth!
Gold is a soft metal, so if you squeeze it hard with your teeth, marks will appear. Agree, the seller will not like it and will force you to buy.
Athletes test their medals for the sake of photos. Moreover, gold medals are actually just gold plated.
By the way, fake aluminum behaves exactly the same way, so if the seller insists on a “teeth test”, don’t be fooled.
On the other hand, only a new product does not have even small scratches on the surface. If a person claims to be selling his used gold, but it appears to have only come from a factory, something is wrong here.
How to determine the density of gold
Real gold can be distinguished from fake gold using a simple but time-tested method. In ancient Greece, it was used by the scientist Archimedes, who was able to detect a fake and convict the jeweler who made the crown for the Syracusan king Hiero from low-grade gold.
Today, massive jewelry is well suited for determining the purity of a precious metal: bracelets, rings, chains, earrings. In order to accurately measure the volume of jewelry, it should not have any separate inserts of other metals or stones.
In order to determine the density of gold jewelry, you must have accurate scales with division values up to a hundredth of a gram and a beaker with volume graduations. The photo below shows step by step a simple method - how to determine the density of gold of any standard.
Among all the chemical elements, gold does not have the highest density; it is significantly ahead of iridium, which has the highest specific gravity among all metals known to people - 22650 kg/m3 or 22.65 g/cm3. Gold is also lighter than three other metals: osmium, platinum and rhenium.
As can be seen from the table above, among the chemical elements there is a metal that is not classified as noble, but has a density very close to gold - this is tungsten . Since tungsten and gold have almost the same density, it is often used by scammers these days. Especially today, counterfeit bars weighing 100 grams or more have become effective. The density of a 999.9 gold ingot is almost identical to the specific gravity of a tungsten ingot. A bank bar with a density of 999 gold is absolutely equivalent in weight to a pure tungsten bar of the same volume and shape.
Another metal that is much rarer, but also still used to make fake gold, is lead . Since the density of gold and lead differs significantly, it will be very difficult for scammers to deceive an attentive buyer of the precious metal. If the gold product is 375 standard, then the specific gravity of gold and lead is almost the same. With high-quality gold plating, lead can easily pass for 375 gold.
Source
STRUCTURE
Crystal structure of gold
Crystallizes in the cubic system, in the form of octahedra, rhombic dodecahedrons, cubes and more complex crystals; They are often distorted, strongly elongated, forming “wires”, “hairs”, or flattened parallel to the octahedron face. Native gold, especially low-grade gold, is characterized by a variety of growth forms; it is usually in the form of skeletal crystals, dendrites, thread-like and twisted-filamentary crystals. Streak-like and irregular lump-like, “hooked” discharges are widespread; Their surfaces often contain imprints of crystals of other minerals, the aggregates of which included accumulations of native gold. Etching reveals the crystalline granular structure of the gold particles.
Units
In D. Mendeleev’s periodic table of chemical elements, this noble metal, as an element, occupies 11th place. The specific gravity of gold is measured in various ways:
Mass and density are interrelated. Provided that the mass of the precious metal is indicated in grams per cubic meter, the same data will also be an analogue of the density of the metal. That is why, knowing the density of gold and silver, you can determine the mass of the precious metal.
The specific gravity of an element can be calculated using a special formula. It looks like this: Y (specific gravity of the element) = P (real weight) / V (density).
Calculation using this formula is used exclusively for gold products. When calculating the mass of other elements, there is likely to be a large error.
Gold is one of the heaviest metals. It ranks seventh among other metals. The density of the yellow precious metal is 19.32 g per cubic centimeter. For clarity, a small ball made of pure noble metal with a diameter of only 4.6 cm has a weight of 1 kg.
Due to the high density of the yellow precious metal, its extraction becomes easy. During the washing process, the rocks are screened out from nuggets and gold sand.
Despite its significant weight , gold in its pure form is very rarely used . This is due to the fact that yellow metal is very soft. Products made from it are easily bent, broken, deformed and do not have sufficient strength. That is why other metals are added to the alloy to increase its hardness. The most commonly used elements are palladium, silver, copper, platinum, nickel and other elements that are not necessarily included in the noble list. The final mass of the product directly depends on what metals and in what quantities are included in the alloy. But the density indicator will remain unchanged.
Price
Noble metal is always dear. Today its value is determined on the stock exchange. There, the units of measurement for elite goods are the troy ounce (denoted as oz) and gram (g).
A troy ounce is 31.1 g. About the same as a gold cube with an edge of just under 1.5 cm.
The price of the gold metal is constantly changing, depending on the state of the economy. If it is “stormy”, it goes up in price. In calm times, business people prefer other financial instruments.
However, noble aurum is a profitable long-term investment. In 20 years, since the beginning of the millennium, it has risen in price sevenfold - from $300 to $2000 per ounce.
Characteristics of individual alloys
The density of a material is a physical property that is determined by the mass of a certain unit of volume. The measurement of the parameter is determined by the ratio of the mass and size of the body.
An alloy of gold with other metals is indicated by a breakdown, which is applied directly to the product.
Rare samples
The highest and rarest standard is 999. It cannot be found on jewelry. This means pure precious metal that is not used for production. The density of 999 gold is 19.3 grams per cubic centimeter.
Slightly more than 96% of pure gold is contained in products marked 958. However, they are extremely rare due to the fragility of the metal and the impossibility of polishing it. The density of this material is 18.3 g/cm3.
900 and 917 samples are rare in Russia. High-quality jewelry is made only to order. Previously, coins were minted from gold metal of the highest standard , which is why the samples are called coin samples. The composition of gold in them is 90%. The alloy contains silver and copper in prescribed proportions. Density indicators for such alloys vary from 17.3 to 17.9 grams per cubic centimeter.
75.5% of the precious metal is contained in products of 750 standard. Additionally, palladium, copper, platinum, silver, and nickel are introduced into the alloy. A fairly durable metal that can be processed, polished and has good strength characteristics. The density of 750 gold varies from 14.4 to 17.4 grams per cubic centimeter.
Most Popular
585 standard (583 in the Soviet Union) contains more than 58% pure yellow metal. The main components of the alloy are silver, nickel, palladium and copper. This is the most popular alloy in jewelry. It is durable, hard, and easy to work with. Most of the jewelry is made from 585 alloy. Its average density is 13 g/cm3.
Products marked 500 contain 50.5% gold. The remaining 49.5% is made up of copper and silver, and not in equal proportions, so the color of the product directly depends on the amount of silver in the alloy. The density of the alloy is 12.3 g per cubic centimeter.
375 standard is considered the cheapest. It contains 38% pure yellow precious metal, the rest is occupied by copper and silver. Low-grade gold tends to deteriorate in appearance when exposed to oxygen and tarnishes over time. The density of the alloy is 11.5 g/cm3.
As a rule, the alloy contains only elements that have a similar specific gravity and density to gold. However, there is a danger of getting a fake instead of gold jewelry. Most often, tungsten is disguised as a yellow precious metal. It has similar performance and appearance. It is extremely difficult to distinguish a fake from the original.
Determination of carat weight
British carats are a traditional value used to measure the purity of gold and its weight in a product. The smallest value is 8 carats (K). The specific gravity of 999 gold is 24 carats. If other elements are added to the alloy to change the characteristics of the metal, the carat value drops. The 18-karat precious metal contains 6 parts of impurities and 18 parts of pure gold.
For example, high-grade 917 gold has a mass of 22 carats. And such a popular 585 alloy is only 14 carats.
Attach a magnet
Plating steel or other alloys with a high iron content with gold or its imitation is a common practice. Only ferromagnets are strongly magnetic - iron, cobalt, nickel, gadolinium, terbium, dysprosium, holmium, erbium, thulium.
Dimagnets (for example, water or table salt) and paramagnets (aluminum, etc.) are weakly magnetic. All non-ferrous metal is not magnetic.
If it happens on the beach or in places where tourists gather, buy the cheapest magnet in a souvenir shop and attach it to the product. If it sticks, it’s definitely a fake or the metal is of too low standard (too many iron impurities). If not, perform other checks.
Gold Assay Density
| gold, silver, copper | 11,54 | 11540 | ||
| pink | gold, silver, palladium, copper | 11,56 | 11560 | |
| 585 | white | gold, silver, nickel, zinc, copper | 12,85 | 12850 |
| gold, silver, palladium | 14,74 | 14740 | ||
| green | gold, silver, copper | 13,92 | 13920 | |
| yellow | gold, silver, copper | 13,70 | 13700 | |
| red | gold, silver, copper | 13,24 | 13240 | |
| 750 | white | gold, silver, nickel, zinc | 15,38 | 15380 |
| gold, silver, palladium | 16,44 | 16440 | ||
| green | gold Silver | 15,96 | 15960 | |
| yellow | gold, silver, copper | 15,45 | 15450 |
Specific gravity of gold and other metals
The specific gravity of gold depends on its purity. There is no perfectly pure gold in the world. Today, with the help of refining processes, the precious metal is most often obtained, the purity of which corresponds to 99.99 % - this approximately corresponds to 1000 samples of gold. Such conditionally pure gold without impurities has a density of 19300 kg/m3 or 19.3 g/cm3. The specific gravity of 999 gold will be slightly less than that of pure yellow precious metal, provided that it does not contain heavier metals.