What is the Mohs hardness scale?
The Mohs scale is a system used to rank materials by their hardness, allowing classification using numbers from 1 to 10. It is used to compare the hardness of gems, metals and certain other materials and evaluate their relative durability.
The Mohs rating of a metal is based on how easily a sample can be scratched by other metals. For example, the hardness coefficient of gold is 2.5-3, which is significantly lower than the hardness coefficient of most other materials.
While graphite and some plastics stand at one end of the scale, having a value of 1, diamond, one of the hardest substances on Earth, is placed at the other end. It is worth 10 points.
Table with comparison scale
Comparison of the strength characteristics obtained using various methods with the Mohs table is useful in the practical use of stones. Absolute values of hardness are determined by other estimates; a comparison of the criteria can be seen in the table.
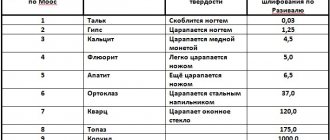
| Minerals and rocks | Mohs grading | According to Schreiner, MPa | Strength on the Knoop scale, units | Grinding hardness in water according to Rozival | Strength according to microhardness tester PMT-2, PMT-3, kg/mm² |
| Talc | 1 | 250―400 | 12 | 0,03 | 2,4 |
| Gypsum | 2 | 32 | 1,25 | 40 | |
| Calcite, marble, anhydrite | 3 | 950―1400 | 135 | 4,5 | 110 |
| Fluorite, dolomite | 4 | 2500―3200 | 160 | 5 | 190 |
| Apatite, granite | 5 | 3000―3700 | 400 | 6,5 | 530 |
| Orthoclase, basalt | 6 | 3900 | 500 | 37 | 790 |
| Quartz, diabase | 7 | 6300 | 1250 | 120 | 1120 |
| Topaz | 8 | 1550 | 175 | 1430 | |
| Corundum | 9 | 1900 | 1000 | 2060 | |
| Diamond | 10 | 8300 | 140000 | 10060 |
Mohs hardness scale for assessing the hardness of metals.
Here is a list of hardness coefficients for some metals that everyone is likely to encounter in their daily life, especially when coming into contact with jewelry:
- Tin: 1.5
- Zinc: 2.5
- Gold: 2.5-3
- Silver: 2.5-3
- Aluminum: 2.5-3
- Copper: 3
- Copper: 3
- Bronze: 3
- Nickel: 4
- Platinum: 4-4.5
- Steel: 4-4.5
- Iron: 4.5
- Palladium: 4.75
- Rhodium: 6
- Titan: 6
- Reinforced steel: 7-8
- Tungsten: 7.5
- Tungsten carbide: 8.5-9
Properties of diamond
The strength of diamond became the standard for the creation of the hardness scale for various minerals, which was developed by Friedrich Mohs. The German scientist gave the stone in question the highest possible score – 10.
• absolute hardness; • high fragility (the material breaks easily); • unique thermal conductivity.
Jewelers value only a few qualities of diamonds - their strong light refraction and pronounced dispersion.
Physicists classify the mineral as a wide-gap semiconductor. This category also includes:
• cadmium sulfite; • silicon carbide; • gallium phosphide.
Another amazing property of the crystal: the minimum coefficient of friction when in contact with metals. If the case occurs in the air, then the indicator is 0.1. The reason is that a gas film forms on the surface of the diamond, which acts as a lubricant.
The hardness of the mineral makes it virtually invulnerable to abrasion, which is why stones are used in drilling equipment.
It is quite possible to burn a diamond - this will require only 1,000 degrees and an air supply. At this temperature, the stone literally evaporates - only carbon dioxide remains. If the mineral is placed in a vacuum and heated to 2 thousand degrees, it will first transform into graphite and then explode.
Why is it important to know the hardness of metals?
When German geologist Friedrich Mohs created the scale we use today, he applied a simple principle to determine the hardness of any material: what materials can scratch it, and what materials it can scratch.
For example, platinum, which has a hardness of 4-4.5, can be scratched by all materials that have a higher Mohs scale. For example, topaz, which has a coefficient of 8, can alternately scratch any material that has a lower coefficient (for example, gold, the hardness of which is rated at 2.5-3 points).
From the table above you can see which metals can scratch others and which ones can scratch them. This is valuable information as it can tell you which precious metals can be stored together and which cannot.
Also, this information about the hardness of metals will help determine which products made from precious alloys are more reliable to wear.
The Hardest. Mohs scale. Chemistry - Simple
How to determine the hardness of a material? Very simple! To do this, you need to use a hardness scale.
The video describes what the Mohs hardness scale is and how to use it. Enjoy watching!
Possible duplicates found
17 minutes is too much)))
The Mohs hardness of a material is the resistance its surface exhibits when someone tries to scratch it with another material. Hardness depends on the degree of cohesion of the intra-atomic structure of the material.
The Mohs scale is a conventional scale of reference minerals for assessing the hardness of materials by scratching. The scale itself is presented below.
Mohs hardness scale
How to apply the hardness scale for metals.
When you decide to buy a product made of a precious metal, but are undecided about which material to choose, the Mohs hardness scale will help.
By comparing the coefficients, you will make a preliminary choice and be able to decide whether this product is also suitable for you in terms of price.
For example, platinum is much more durable than silver, and in general, harder ones last longer with constant wear. However, platinum is also much more expensive than silver, so you need to consider whether you are willing to pay the extra price for durability.
NOT ONE BLOOD
Oddly enough, the most accessible material for protecting dials has nothing to do with glass - at least at the molecular level. Plastic, which is called organic glass, sometimes plexiglass, is actively used in various inexpensive mechanical and electronic watches. The material under the Plexiglas brand was created in 1928, and its industrial production began in 1933. The appearance of plexiglass in the period between the two world wars was not accidental, and the engine of its spread was the development of aviation, which required a combination of strength, optical transparency, shatterproofness, that is, safety for the pilot, resistance to moisture and technical fluids.
Organic glass consists entirely of thermoplastic resin, and the production process is represented by two main methods: extrusion (extrusion of a hot polymer mass through a gap of a certain width and thickness) and casting (when a hot polymer mass is poured between two layers of glass or metal, and the size of the gap between the sheets is determined thickness of the future sheet).
Plastic glass is easy to scratch, but not so easy to break
The main advantage of plastic for the watch industry is its ability to easily take any shape, obediently following the will of designers. The relative softness is also reflected during use: plexiglass is easy to scratch, but not so easy to break. Plexiglas is easy to manufacture and maintain: polishing or replacing such glass is easy and inexpensive. It is a pity that it will have to be changed or polished often, especially if the owner of the watch leads an active lifestyle.
Hardness of metal alloys.
The Mohs scale for each metal indicates the hardness in its pure state, i.e. without any other materials mixed with it.
However, in reality, almost all metals used in jewelry are combined with others to create a stronger or cheaper material.
For example, gold is often mixed with nickel, zinc, copper and other metals to give it extra hardness.
Likewise, when carbon is added to tungsten, which has a hardness rating of 7.5 in its pure form, the resulting tungsten carbide will have a hardness rating of 8.5-9 on the Mohs Hardness Scale.
Mineral hardness test kit
Linear hardness
Linear hardness is determined by the absolute hardness scale, not the Mohs scale. Here is the absolute hardness scale:
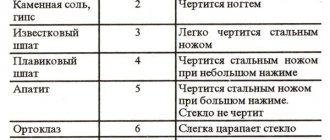
Talc – 1 – Can be scratched with a fingernail Gypsum – 3 – Scratched with a fingernail Calcite – 9 – Scratched with a copper coin Fluorite – 21 – Easily scratched with a knife Apatite – 48 – Hardly scratched with a knife Orthoclase – 72 – Scratched with a file Quartz – 100 – Scratches window glass Topaz – 200 – Easily scratches quartz Corundum – 400 – Easily scratches topaz Diamond – 1600 – Not scratched by anything (and at the same time easily scratches corundum)
What is the hardness of steel? (Part 1)
The concept of metal hardness was previously known only to graduates of technical universities, workers of machine-building plants and blacksmiths. This term came into use with the modern knifeman with the adoption of the law on weapons and GOST standards, which provide signs on the basis of which a knife can be classified as a bladed weapon.
One of the mandatory characteristics by which a particular product is classified as a bladed weapon is the hardness of the steel from which the knife blade is made (or, as it is called in GOST, the warhead of a bladed weapon). And starting from this moment, the nyfomaniacs in Russia began to slowly read reference books that provide the characteristics of different steels, explanations of the differences between powder and laminated steels, and of course the steel hardness indicators, those very noticeable HRC.
If one car enthusiast can ask another about how many “cubes are in the engine,” then an advanced knifomaniac, looking at the characteristics of a field knife that says “57-59 HRC,” can seriously determine that this is a flimsy model for bushcraft and it belongs on kitchen.
This article will tell you in a simple and understandable form what kind of beast this HRC is, where it came from and why it is needed at all.
Interesting fact: On American and European websites, among the parameters indicated by sellers or manufacturers, it is extremely rare to find such a parameter as steel hardness.
This issue is not regulated in any way by law, so the average inexperienced buyer does not need this parameter.
So, what do we need to know about the hardness of metals?
Since ancient times, man has encountered the concept of hardness of materials. I also quickly realized that different materials differ from each other in hardness and strength. If you hit a stone with a stick, the stick will either break or bounce off. If you hit a stick with a stone, the stick will break. If a coconut falls from a tree onto a pebble beach, it will break. And if you hit a softer stone with flint for a long time and diligently, then you can quite easily make a head for a stone axe.
Gradually, in the process of evolution, our ancestors realized that different materials have different hardness, and depending on this hardness, they do or do not have the desired properties. Thus was born a method for determining the hardness of a material by comparing it with a certain standard.
Thus, a good carpenter can determine the degree of shrinkage of a log by tapping it with a mallet made of a harder type of wood. Using a special hammer, a potter can determine the degree of readiness of pottery. Willingly or unwittingly, each of us at least once in our lives has resorted to a similar method of determining the hardness of an object.
However, until recently, the most common method for determining the hardness of a material was the sclerometric method. Sclerometry is a physical process where the material being tested scratches (or scratches) a reference sample. If the material being tested scratches the standard, it means the material being tested is harder.
If the material being tested cannot leave a mark on the standard and is easily scratched by the standard, then the material being tested has a hardness less than that of the standard. Now this procedure seems ridiculous to us, but until recently, this was the only way to determine the hardness of a material. How else could the ancient Sumerians determine that it was possible to write inscriptions with a sharp stick on almost dried clay?
The issue of determining the hardness of materials (especially stones and metals) became acute at the end of the 18th and beginning of the 19th centuries, with the development of geology and the beginning of the flowering of mechanical engineering.
It was at this time that the “Mohs scale”, known to all physicists and archaeologists, appeared. However, the first to propose measuring the hardness of metals by comparing them with a standard was the French naturalist of the mid-18th century, Rene Antoine Reaumur.
Reaumur actively conducted experiments related to the melting and processing of metals and therefore he was faced with the acute question of determining the various characteristics of the alloys that he obtained in the process of his research.
His ideas were picked up and developed by the German naturalist and geologist Karl Friedrich Christian Mohs. In 1811, he came up with a system for standard comparison of minerals, which now bears his name. Until about the middle of the 20th century, this scale was actively used by geological exploration parties around the world.
The Mohs scale is a comparative table in which known minerals of different hardness are indicated and their hardness is measured in the following criteria:
- Scratched with a fingernail;
- Scratched by copper;
- Scratched by glass;
- Scratches glass;
- Processed only with diamond.
Tal is the softest reference mineral, while diamond is the hardest mineral. The hardness of talc on the Mohs scale is “1”, the hardness of diamond is “10”. Between talc and diamond, as hardness increases, there are: gypsum (hardness 2), calcite (hardness 3), fluorite (hardness 4), apatite (hardness 5), orthoclase (hardness 6), quartz (hardness 7), topaz (hardness 8 ), corundum (hardness 9). This simple method of determining the hardness of minerals turned out to be indispensable in field conditions.
In addition to the Mohs scale, there are other methods for determining the hardness of materials, which were actively developed at the end of the 19th and beginning of the 20th centuries. There are usually four most well-known methods for determining the hardness of metals:
- Brinell method;
- Vickers method;
- Shore method;
- Rockwell method.
Looking ahead, we note: all these methods are similar to each other, since they are based on pressing a reference sample into the metal surface. Only the shape of the standard, the pressure force, and the formula for calculating the value differ.
The element that is pressed into the surface of the metal is called an “indenter”. A steel ball (Brinell method), a diamond cone (Rockwell method), or a diamond pyramid (Vickers and Shore methods) can be used as an indenter.
Origin of stones
Diamond is an Arabic corruption of the Greek word “adamas,” which translates as “indestructible.” This term refers to a mineral that is an allotrope of the most common carbon.
The stone has an amazing and extremely rare property - metastability. Simply put, in its natural environment it is capable of existing for an unlimited period of time. So the expression “diamonds are forever” is completely true. However, if you place the crystal in a chamber filled with any inert gas and start heating it, it will turn into penny graphite.
Obsidian has been known to mankind much longer than diamond. It has many alternative names, the most common being:
• volcanic glass; • devil's claw.
Mechanical methods for determining hardness.
The hardness of a material is the ability to resist mechanical penetration of another hard material into its surface layer. It is determined by the magnitude of the load required to begin the destruction of the material. Hardness is divided into relative and absolute. Relative hardness is the hardness of one material relative to another. Absolute hardness is determined using indentation methods.
Hardness depends on many factors. Among them: interatomic distances of a substance, valence, the nature of chemical bonds, fragility and malleability of the material, flexibility, elasticity, viscosity and other qualities.
The hardest materials existing today are two allotropic modifications of carbon - lonsdaleite, which is one and a half times harder than diamond, and fullerite, which is twice as hard as diamond. However, among the common substances, diamond is still the hardest.
There are several scales (measurement methods) for measuring hardness. They will be different for different materials. The following methods are used to measure the hardness of metals:
Brinell method
— hardness is determined by the diameter of the imprint left by a metal ball pressed into the surface. Hardness is calculated as the ratio of the force applied to the ball to the area of the indentation.
There are two types of hardness calculation methods:
Using the reconstructed indentation method, hardness is calculated as the ratio of the applied load to the surface area of the indentation:
,
- — applied load, H;
- — ball diameter, mm;
- — imprint diameter, mm.
According to the unrestored indentation method, hardness is determined as the ratio of the applied load to the area of the part embedded in the material and the indenter:
,
where is the penetration depth of the indenter, mm.
Units of measurement are kgf/mm². The hardness determined by this method is denoted by HB, where H = hardness, B - Brinell. These are some of the oldest methods, used back in the 19th century.
Rockwell method
— hardness is determined by the relative depth of indentation of a metal or diamond cone into the surface of the material being tested. Hardness is denoted by HR, where H is hardness and R is Rockwell. Hardness is calculated by the formula HR = 100 − kd, where d is the depth of indentation of the tip after removing the main load, and k is the coefficient. Thus, the maximum Rockwell hardness corresponds to HR 100. The 3rd letter in the designation is the name of the scale type, for example. HRA, HRB, HRC, etc. For knives, hardness is determined according to the HRC scale, which actually ends at 70 units, since the high hardness of a knife does not allow them to be fully used due to a decrease in toughness, increased fragility, etc. This system was the most common in the 20th century.
Rockwell hardness can be measured:
1) Diamond cone with a total load of 150 kgf. Hardness is measured on the C scale and is designated HRC (for example, 62 HRC). The method allows you to determine the hardness of quenched and tempered steels, materials of medium hardness, surface layers with a thickness of more than 0.5 mm;
2) Diamond cone with a total load of 60 kgf. Hardness is measured on the A scale, which is the same as the C scale, and is designated HRA. Used to evaluate the hardness of very hard materials, thin surface layers (0.3 ... 0.5 mm) and thin sheet material;
3) A steel ball with a total load of 100 kgf. Hardness is designated HRB and is measured on the B scale. This is how the hardness of mild (annealed) steel and non-ferrous alloys is determined.
When measuring hardness using a Rockwell instrument, it is necessary that the surface of the sample is free of scale, cracks, gouges, etc. It is necessary to control the perpendicularity of the load applied to the surface of the sample and the stability of its position on the instrument table. The indentation distance must be at least 1.5 mm when pressing the cone and at least 4 mm when pressing the ball. Hardness is measured at least 3 times on one sample, then the average value is displayed. The advantage of the Rockwell method over the Brinell and Vickers methods is that the hardness value according to the Rockwell method is recorded directly by the indicator needle, eliminating the need for optical measurement of the indentation dimensions.
Vickers method
- the widest scale in scope, hardness is determined by the area of the imprint left by a tetrahedral diamond pyramid pressed into the surface. It is designated HV, where H is Hardness, V is Vickers. When testing hardness using the Vickers method, a diamond tetrahedral pyramid with an angle is pressed into the surface of the material. After removing the indentation load, the diagonal of the indentation is measured. The Vickers hardness number is designated by the symbol HV indicating the load P and the holding time under load, and the dimension of the hardness number (kgf/mm2) is not specified. The duration of exposure of the indenter under load is 10–15 s for steels, and 30 s for non-ferrous metals. The advantages of the Vickers method over the Brinell method are that the Vickers method can test materials of higher hardness due to the use of a diamond pyramid.
Shore hardness
(Indentation method)
- hardness is determined by the depth of penetration into the material of a special hardened steel needle (indenter) under the action of a calibrated spring. This measurement method uses a device called a durometer. Typically, the Shore method is used to determine the hardness of low-modulus materials (polymers). Shore's method involves 12 measurement scales. The most commonly used options are A (for soft materials) or D (for harder materials). The hardness determined by this method is indicated by the letter of the scale used, written after the number indicating the method. As an example, the rubber in a passenger car tire has a hardness of approximately 70A, and a school eraser has a hardness of approximately 50A.
Shore Hardness (Rebound Method)
- a method for determining the hardness of very hard materials, mainly metals, by the height to which, after an impact, a special striker falling from a certain height rebounds. Hardness according to this Shore method is assessed in conventional units proportional to the height of the striker’s rebound. It is designated HSx, where H is Hardness, S is Shore and x is a Latin letter indicating the type of scale used in the measurement.
Libu method (hardness testers)
This is the most widely used method in the world today, hardness is defined as the ratio of the velocities before and after the striker rebounds from the surface. It is designated HL, where H is Hardness, L is Leeb, and the 3rd letter indicates the type of sensor, for example. HLD, HLC, etc. When using this method, a striker falling normally to the surface of the material under study collides with the surface and rebounds. The speed of the striker is measured before and after rebound. It is assumed that the firing pin is not subject to permanent deformation.
Asker method
— hardness is determined by the depth of insertion of the steel hemisphere under the action of a spring. Used for soft rubbers. According to the measurement principle, it corresponds to the Shore method, but differs in the shape of the probe surface. Asker uses a hemisphere with a diameter of 2.54 mm.
Kuznetsov-Herbert-Rehbinder method
— hardness is determined by the damping time of the oscillations of the pendulum, the support of which is the metal under study.
Poldi method (double ball imprint)
— hardness is assessed in comparison with the hardness of the standard, the test is carried out by impact pressing a steel ball simultaneously into both the sample and the standard.
Hardness of minerals.
Mohs mineral hardness scale
(scratching sclerometers) - a method for determining the hardness of minerals by scratching one mineral with another, for comparative diagnosis of the hardness of minerals among themselves according to the softer-harder system. The mineral being tested is either not scratched by another mineral (Mohs standard or sclerometer) and then its Mohs hardness is higher, or it is scratched - and then its Mohs hardness is lower. Mohs scale - determines which of ten standard minerals scratches the test material, and which of ten standard minerals scratches the test material.
Methods for measuring hardness
The degree of strength of a material is assessed by pressing a stronger object into it, as well as grinding and scratching its surface. The choice of which scale to measure the hardness of minerals is large. Besides leaving a groove with Mohs reference crystals, there are 6 main measurement methods:
- Rockwell scale - the depth of penetration of an identifier with a diamond tip into the material is recorded. Applicable to metals and alloys.
- Shore hardness is determined in the same way. Additionally, the method allows you to study the strength of plastic and elastic objects.
- The Knoop scale works on the principle of indentation. The result is assessed in Knoop units: diamond – 8500, corundum – 2000.
- Rock hardness using the Schreiner method is quantitatively determined by the ratio of the load on the punch to the die area. The measurement is accompanied by drawing a deformation diagram.
- The Rozival scale is based on the example of the Mohs table: indicators for a number of minerals were obtained using a hardness tester based on the results of grinding samples. The complexity of the method did not allow it to displace the classics.
- A Vickers pyramid reinforced with diamonds is statically pressed into the test object, and the result is viewed from the area of the indentation through a microscope. The devices are called hardness testers (for example, PMT-3).
All accurate methods for finding strength are inferior to the quick tabular method of determining by comparing the strength properties of minerals. It is not always necessary to see the breaking load; in most cases, it is enough to know which stone is harder.
Mohs hardness scale
The Mohs scale (mineralogical hardness scale) is a qualitative ordinal scale that characterizes the scratch resistance of various minerals. Used to determine the relative hardness of mineral samples.
Based on the ability of a harder material to scratch a softer material.
The scale contains 10 minerals as reference minerals, ranking them in order of increasing hardness from very soft (talc) to very hard (diamond).
All of the minerals in the table, except diamond, are relatively common and easy or inexpensive to obtain.
If a mineral scratches a standard, then its hardness is higher; if it is scratched by a standard, it means lower.
The Mohs scale was created in 1812 and named after its inventor, German geologist and mineralogist Friedrich Mohs. Since then, many different methods for determining hardness have been invented: the Brinell, Knoop, Rockwell, Shore, Vickers method.
Mohs hardness is a relative integer comparison of scratch resistance.
Other hardness measurement methods rely on indentation resistance. For testing, an “Indenter” is used, which is pressed into the test sample with a carefully measured force. The size or depth of the notch in the specimen and the magnitude of the force are then used to calculate the hardness value. Because each of these tests uses different apparatus and different calculations, they cannot be directly compared to each other.
The Mohs scale has become widespread because... The hardness test method is easy to perform, inexpensive and quickly understood by people.
Despite its lack of accuracy, the scale is useful for field geologists who use it for rough identification of minerals when examining easily identifiable samples or when more complex tests are not available.
Some use readily available items for a quick test. For example, a geologist may have a pocket knife that can be used to determine whether a sample is harder or softer than a Mohs value of 5-6.5.
Below is an extended table of substances, minerals, and precious stones:
| Substance or mineral | Mohs hardness |
| Pyrophyllite, molybdenite | 1-2 |
| Bauxite, coal | 1-3 |
| Limonite | 1-5 |
| Ice, sugar, gallium, strontium, indium, tin, barium, thallium, lead, graphite | 1,5 |
| Gypsum, calcium | 1,5-2 |
| Sulfur | 1,5-2,5 |
| Sylvite, glauconite, cadmium, selenium | 2 |
| Rock salt, cinnabar, chlorite, bismuth, amber | 2-2,5 |
| Muscovite | 2-3 |
| Silver, gold, galena, copper, biotite, mica | 2,5-3 |
| Aluminum, limestone, calcite, boric acid, nitrophoska | 3 |
| Aragonite, witherite, anhydrite | 3-3,5 |
| Pearl, brass, arsenic | 3-4 |
| Serpentine | 3-5 |
| Sphalerite, rhodochrosite, malachite, dolomite, cuprite, chalcopyrite, azurite, barite | 3,5-4 |
| Siderite, pyrrhotite, dolomite | 3,5-4,5 |
| Fluorite, phosphor bronze | 4 |
| Marble | 4-5 |
| Tooth enamel, asbestos, apatite, manganese, zirconium, palladium, obsidian | 5 |
| Titanite, monazite | 5-5,5 |
| Jade, uraninite, ilmenite, enstatite, porcelain stoneware (polished) | 5-6 |
| Magnetite | 5-6,5 |
| Nepheline, augite, arsenopyrite, actinolite, bustamite, cobaltite | 5,5-6 |
| Rhodonite, diopside, opal, red iron ore | 5,5-6,5 |
| Titanium, germanium, niobium, rhodium, uranium | 6 |
| Rutile, pyrite, prehnite, plagioclase, orthoclase, amazonite, andesine, anorthoclase, benitoite, helvite, iridium | 6-6,5 |
| Silicon | 6,5 |
| Jasper | 6,5-7 |
| Agate, zoisite, epidote, cassiterite, pyrolusite | 6-7 |
| Marcasite | 6-7,5 |
| Granite, tanzanite, spodumene, olivine, jadeite, axinite, chrysoprase, jadeite | 6,5-7 |
| Sillimanite, garnet | 6,5-7,5 |
| Quartz, stone pebbles, amethyst, aventurine, forsterite, osmium, silicone, rhenium, vanadium | 7 |
| Tourmaline, cordierite, almandine, boracite, cordierite, danburite | 7-7,5 |
| Zircon, andalusite, euclase, hambergite, sapphirine | 7,5 |
| Emerald, hardened steel, tungsten, spinel, beryl, beryllium, aquamarine, red beryl, ganite, painite | 7,5-8 |
| Topaz, cubic zirconia | 8 |
| Chrysoberyl, alexandrite, choltite | 8,5 |
| Porcelain tiles (unpolished) | 8,5 |
| Corundum, ruby, sapphire, alundum, chrome | 9 |
| Moissanite, boron | 9,5 |
| Carborundum | 9-10 |
| Diamond, carbonado | 10 |
© 2014-2020 All rights to materials on the site are protected in accordance with the legislation of the Russian Federation.
14.10.8.1. Mineral pigments and fillers
Table 14.87
White pigments
| Name | Formula | Standards | Color Index | Crystal structure | Hardness according to the Mooca scale, y. e. | Density, 103 kg/m3 | Specific surface area, m2/g | Refractive index, vacuum/alkyd | Whiteness, u. e. | Covering capacity, g/m2 | Oil capacity, g/100 g | Heat resistance, °C | Chem. durability | |
| OH– | H+ | |||||||||||||
| 1. Titanium dioxide, titanium dioxide, titanium white | TiO2 | GOST 9808–84 ASTM D476 ISO 591 DIN 55912 BS 1851 | PW 6 (77891) | Tetragonal, anatase | 5,0–6,0 | 3,7-4,1 | 6,0–15,0 | 2,55 /1,65 | 96–97 | 32–45 | 20–30 | 250–300 | + | + |
| Tetragonal, rutile | 6,0–7,0 | 3,7-4,2 | 5,0–20,0 | 2,76 / 1,75 | 94–96 | 30–40 | 16–25 | 200–300 | + | + | ||||
| 2. Zinc oxide, zinc oxide, zinc white | ZnO | GOST 202–84 ASTM D 79 DIN 55943 BS 254 | PW 4 (77947) | Hexagonal | 4,0–4,5 | 5,6 | 3–10 | 2,0 / 1,29 | 95–97 | 110–140 | 12–20 | 400–700 | + | – |
| 3. Zinc sulfide | ZnS | DIN 55910 BS 1316 | PW 7 (77975) | Hexagonal (wurtzite) | 3,0 | 4,0–4,1 | 6,0–8,0 | 2,37 / 1,53 | 98 | 35 | 13–14 | 600 | + | – |
| 4. Lithopone | ZnS + BaSO4 | GOST 907–72 ASTM D3280 ISO 473 DIN 55910 BS 239-296 | PW 5 (77115) | ZnS - hexagonal BaSO4 - orthorhombic | ZnS - 3.0 BaSO4 - 3.5 | 4,1–4,3 | 3,0–5,5 | 1,84 / 1,19 | 90–94 | 110–140 | 11–15 | 600–700 | + | – |
| 5. Basic lead carbonate, white lead | 2PbCO3 · · Pb(OH)2 | TU 6-33-501-1902-102–90 ASTM D 81 BS 239 | PW 1 (77597) | Hexagonal | 6,4–6,8 | 2,7 | 1,94 / 1,29 | 95 | 160–220 | 9–14 | 200 | – | – | |
| 6. Basic lead sulfate, sulfate lead white | 2PbSO4 · · Pb(OH)2 | BS 637 ASTM D 82 | PW 2 (77633) | 6,4–6,7 | 2,5 | 1,93 / 1,30 | 95 | 90 | 8–10 | 200 | – | – | ||
Table 14.88
Fillers
| Name | Formula | Standards | Hardness on the Mohs scale, y. e. | Density, 103 kg/m3 | Specific surface area, m2/g | Average particle size, microns | Refractive index vacuum/alkyd | Whiteness, u. e. | Oil content-bone, g/100 g | Heat resistance, °C | pH | Chem. durability | |
| HE- | H+ | ||||||||||||
| 1. Natural calcium carbonate - calcite, chalk | CaCO3 | GOST 1285–88 ASTM D1199 DIN 55918 BS 1795 | 3,0 | 2,7 | 1–50 | 1,48–1,96/ /0,95–1,06 | 80–85 | 12–22 | 300 | 8–10 | + | – | |
| 2. Precipitated calcium carbonate | CaCO3 | TU 5743-003-05346-453–97 DIN 55918 | 3,0 | 2,65–2,70 | 0,05–0,35 | 1,49–1,69/ /1,01 | 95–97 | 28–58 | 300 | 9–10 | + | – | |
| 3. Dolomite, calcium and magnesium carbonate | n CaCO3 · MgCO3 n = 1.18¸1.24 | GOST 236-72–79 DIN 55919 | 3,0 | 2,75–2,90 | 1,0–3,0 | 2–30 | 1,50–1,68/ /1,03 | 84–96 | 14–20 | 300 | 8–10 | + | – |
| 4. Natural barium sulfate, barite, feldspar | BaSO4 | TU 4682–84 ASTM D602 DIN 55911 BS 1795 | 3,0–3,5 | 4,4–4,5 | 2,0–3,0 | 2–10 | 1,64/1,06 | 72–92 | 10–11 | 600 | 6–10 | + | + |
| 5. Synthetic barium sulfate, blancfix | BaSO4 | GOST 11120–75 ASTM D602 DIN 55911 BS 1795 | 2,5–3,5 | 4,1–4,5 | 2,0–4,0 | 0,7–6 | 1,64/1,06 | 95–100 | 12–40 | 600 | 6–10 | + | + |
| 6. Natural silicon dioxide: amorphous (diatomite, kieselguhr) | SiO2 72–88% | STP ZHK 001–96 ASTM D604 DIN 55630 BS 1795 | 6,0 | 1,9–2,3 | 20 | 3–60 | 1,42–1,48/0,94 | 75–88 | 120–130 | 200 | 7–9 | + | + |
| crystalline (quartz) | SiO2 99% | GOST 9077–82 DIN 55926 | 7,0 | 2,65 | 15–150 | 1,55/1,01 | 95–98 | 13–30 | 7 | + | + | ||
| 7. Silicon dioxide: synthetic silica gel | SiO2 | DIN 55921 | 6,0 | 2,0–2,2 | 130–400 | 0,07–4,0 | 1,46/0,94 | 81–96 | 145–350 | 200 | 3,5–8 | + | + |
| Aerosil | GOST 14922–77 | 6,0 | 2,2 | 150–320 | 0,007–0,016 | 1,45/1,55 | 280–300 | 2,2 | + | + | |||
| 8. Kaolin, natural aluminum silicate | Al2O3 2SiO2 2H2O | GOST 21285–75 ASTM D602 DIN 55922-A, B | 2,5 | 2,58–2,63 | 13–20 | 0,5–7,0 | 1,56/1,0 | 80–90 | 40–55 | 400 | 4,5–6 | + | – |
| 9. Natural aluminum silicate, bentonite, colloidal clay | Al2O3 · SiO2 · 2H2O, impurities: Fe2O3 - 1.7%, MgO - 2% Na2O + K2O - 2.2% | TU 6-1282–79 DIN 55922-C, D | 2–5 | 1–8 | 13–20 | 0,5–5,0 | 1,58–1,61/ /1,02–1,04 | 50–90 | 50–60 | 250 | 4–9 | + | + |
| 10. Talc, natural magnesium silicate | 3Mg 4SiO2 H2O | ASTM D605 DIN 55924 | 1,0 | 2,6–2,8 | 8–10 | 20–90 | 1,54–1,59/ /0,99–1,03 | 70–90 | 30–50 | 500 | 8–9,5 | + | + |
| 11. Wollastonite, calcium metasilicate, plank spar | CaSiO3 | 4,5–5,0 | 2,8–2,9 | 4,0–8,0 | 20–60 | 1,63/1,04 | 85–90 | 20–26 | 600 | 8–12 | + | + | |
| 12. Synthetic calcium silicate | CaSiO3 nH2O _ | DIN 55921 | 1,9 –2,4 | 8,0–10,0 | 1,55–1,63/ /1,00–1,05 | 93 –95 | 600 | 8 –12 | + | – | |||
| 13. Mica, potassium or magnesium aluminum silicate: muscovite | K2O 3Al2O3 6SiO2 2H2O | GOST 10698–80 ASTM D607 DIN 55607 BS 1795 | 2,74–2,88 | Plates with a diameter of 5–150 µm | 1,58–1,59/ /1,50–1,55 | 70–80 | 20–50 | 600 | 7–9,5 | + | + | ||
| flagopite | 3MgO 1.5Al2O3 8SiO2 9H2O | 2,5 | 2,36 | 1–5 | 1000 | + | – | ||||||
| 14. Aluminum hydroxide, transparent white | Al(OH)3 | TU 1711-001-00658716–99, TU 48-5-128–89 ISO 1247 ASTM D962 DIN 55628 BS 1795 | 2,0–2,3 | 60–70 | 0,1–1,0 | 1,54–1,57 | 88–93 | 80–150 | 120 | 7–9 | – | – | |
Table 14.89
Synthetic chromatic pigments
Notes : 1. The optimal pigment particle size, which ensures better light scattering in the pigment dispersion, and therefore the highest color brightness, high coloring power and hiding power, is 0.16–0.28 microns. However, due to the agglomeration of particles and differences in manufacturing techniques (synthesis and grinding processes), the dispersed composition of pigments usually differs from the optimal one and may vary depending on the production method. Therefore, indicators such as specific surface area, oil absorption, and hiding power can vary within certain limits for each pigment and do not have a stable value. The tables show average values for all pigments. 2. New pigments (Table 14.91). In addition to the colored synthetic pigments listed in the table, a large group of colored zirconium pigments has become widespread in the last decade. They are obtained by introducing 1–3 ions into the zirconium crystal lattice - ZrSiO4-. The tetragonal structure of zirconium silicate is defective and allows elements to be incorporated into it, resulting in colored compounds. In addition, zirconium silicate has the ability to form mixed products such as core pigments with a number of pigments, creating protective shells and providing the resulting products with high thermal and chemical resistance. Zirconium pigments are used primarily as ceramic pigments for high temperature glazes, enamels and in porcelain production.
In table Figure 14.90 shows the dependence of the properties of iron oxide pigments (α-Fe2O3) on particle size.
Table 14.90
Dependence of the properties of iron oxide pigments on particle size
| Properties | Particle size, microns | ||
| 0,001–0,01 | 0,1–1,0 | 10–100 | |
| Type of pigment | Transparent iron oxide pigment | Opaque iron oxide pigment | Iron mica |
| α-Iron Oxide Shade | Yellow-red | From yellow-red to purple | Gray-brown with a metallic sheen |
| Covering power | Transparent (glaze) pigment | High coverage pigment | Low coverage pigment |
| Tendency to settle | |||
| Specific surface area | |||
| Flocculation ability | |||
| Dispersibility | |||
| Oil capacity | |||
Note : Arrows indicate a qualitative trend of increasing or decreasing indicator.
New color pigments that can replace toxic red pigments - cadmium and lead - include cerium sulfides, which are already being produced in limited quantities. Ce2S3 orange, CIPO75 (77288:1) and Ce2S3 red CIPR265 (77288:2). Their density is 5000 kg/m3, their hiding power is close to that of cadmium pigments, the color is less bright, and heat resistance is 290–320 °C. Chemically they are less stable, decomposed by hot water and weak acids. They are easily dispersed and can be used for coloring plastics and in the production of paints.
Yellow pigments that have recently been developed include bismuth vanadate BiVO4.
Color - bright yellow, CIPY 184, density - 7500 kg/m3, refractive index - 2.45, specific surface - 5–15 m2/g, heat resistance - 200 ºС. Used in the paint and varnish industry and for painting plastics.
Table 14.91
Colored pigments based on zircon
| Elements built into the grille | Color | Pigment in zircon shell | Color |
| Xie, Eu | Pink | Fe2O3 | Coral |
| Cd(S, Te) | Pink | ||
| Pr, Mg | Orange-red | Cd(S,Se) | Red |
| Cd, Hg(S, Se) | Orange | ||
| Ce, Pr, Mg | Orange-red | (Cd,Zn)S | Yellow |
| CdS | Yellow | ||
| PbCrO4 | Yellow | ||
| Ce, Pr | Yellow-orange | TiO2 NiSbO4 | Yellow |
| Pr | Yellow | Cu compounds | Green |
| Pr, Mo | Yellow | (Co, Zn)Al2O4 | Blue |
| Tb | Yellow | Au | Blue-violet |
| Ce, Tb | Yellow | Au, Ag, Zn | Red-violet |
| Ce,Dy | Yellow | PbS | Grey |
| Ce | Ivory | SnS | Grey |
| Ni | Yellow-green | MoS2 | Grey |
| V, Mn | Blue-green | Mo(S, Se)2 | Grey |
| Cr | Green | VS | Grey |
| V | Blue-green | ||
| Cu | Blue-green | ||
| Co | Blue-green | ||
| Ce,Nd | Blue-violet | ||
| Nd | Light purple | ||
| Cr, Co, Cu | Dark purple | ||
| Ni, Co | Grey | ||
| Mn | Grey, pink |
Table 14.92
Natural pigments based on iron oxides
Note
.
Iron mica, unlike other pigments, has plate-like particles 5–100 µm wide and 2–5 µm thick.
Table 14.93
Natural pigments with a different color base
Anti-corrosion pigments
Anti-corrosion pigments perform the function of corrosion inhibitors in coatings, and according to the mechanism of protective action they are divided into 3 groups:
- pigments that provide chemical protection (they contain soluble components and, due to chemical reactions, maintain a constant pH in the coating);
- pigments that provide electrochemical protection: some of them prevent corrosion by forming a protective coating and are active in the anodic region of the metal surface (anodic protection), others prevent corrosion due to oxidation potential and are active in the cathodic region (cathodic protection);
- pigments that are chemically inert, but create mechanical barriers to the penetration of moisture and aggressive gases (“barrier effect”).
The previously widely used lead-containing anti-corrosion pigments (minimum lead, lead cyanamide, etc.) due to their high toxicity are completely replaced by a group of chromates, phosphates and other pigments, the properties of which are described below.
CHROMATS: have low solubility, provide cathodic protection and form a protective film as a result of the interaction of chromate ions with the metal surface.
PHOSPHATES: provide anodic protection, form a protective coating on the metal surface.
MOLYBDATES: provide anodic protection. They are expensive and are usually combined with phosphates, zinc white, calcium and magnesium carbonates.
CALCIUM PLUMBATE: is chemically active, forming calcium hydroxide in the coating and raising the pH to 11-12.
Calcium, zinc FERRITES: chemically active, forming calcium hydroxide in the coating and increasing the pH.
ZINC DUST: chemically active (forms zinc hydroxide and increases pH).
IRON MICAS and other flake pigments create a “barrier effect”. Mica, talc, aluminum powder, etc. are used as such pigments.
ION EXCHANGE compounds are active anti-corrosion pigments that have a chemical effect: calcium ions of these compounds exchange in the film with hydrogen ions of the environment (neutralize acidity), and then bind to oxides on the metal surface.
The table below shows the main properties of anti-corrosion pigments.
Table 14.94
Anti-corrosion pigments
Table 14.95
New promising phosphate-type anti-corrosion pigments and their areas of application
| Composition (formula) | Film former | Metal-substrate (substrate) |
| CaZn2(PO4)2 2H2O | Alkyd, chlorinated rubber | Iron |
| CaHPO4 2H2O/ZnO | Polyvinyl butyral, phenol-formaldehyde oligomer, alkyd | Aluminum, iron |
| MgHPO4 3H2O/ZnO | Alkyd, polyvinyl butyral, phenol-formaldehyde oligomer | Iron, aluminum |
| CaHPO4 2H2O/MgHPO4 3H2O | Alcides | Iron |
| (0.5MgHPO4 0.5MgCO3 ) xH2O | Epoxy, polyamide | Aluminum |
| (0.4SrHPO4 0.6SrCO3) x H2O + (0.1–3)% F | Epoxy, polyamide | Aluminum |
| TiO2/ZrO/SiO2 x P2O5 | Alkyd-melamine-formaldehyde oligomer | Iron |
| (F x , Cr y , Na z )PO4 x H2O | Epoxide | Iron |
| Al(H2PO4)3 x H2O/ZnO | Alcides | |
| (Ca, Mg)3(PO4, MoO4)2 x H2O | Epoxy, alkyd-melamine-formaldehyde oligomer | Iron |
| Zn3(PO4)2 (2–4)H2O x ZnMoO4 | Alcides | Iron |
| (Zn, Ca)3(PO4, MoO4)2 x H2O | Alkyd, epoxy | Iron |
Table 14.96
Possibility of using anti-corrosion pigments in soils of various natures
| Anti-corrosion pigments | Natural drying alkyd primers | Alkyd-melamine primers | Two-component epoxy primers | Two-component acrylic isocyanate primers | Soils based on chlorkau-chuk | Aqueous polymer dispersions | Primers based on polyvinyl butyral | Electro-deposited coatings |
| Zinc phosphate | + | + | + | + | + | + | O | O |
| Chromium phosphate | + | O | – | – | – | – | – | O |
| Aluminum phosphate | + | + | + | – | + | + | + | – |
| Zinc phosphomolybdate | + | + | + | – | + | + | + | O |
| Calcium-zinc phosphomolybdate | + | – | + | – | – | + | – | + |
| Zinc hydrophosphite | + | – | + | – | + | + | – | – |
| Barium metaborate | + | + | – | – | – | + | – | – |
| Zinc borophosphate | O | – | O | – | – | + | + | – |
| Zinc tetraoxychromate | + | + | – | – | – | + | + | – |
| Strontium chromate | + | + | + | – | – | – | + | + |
Note
.
“+” - good anti-corrosion properties, often used;
“o” - satisfactory anti-corrosion properties; “–” are rarely used. Table 14.97
Metal pigments
| Name | Formula | Standards | Density, 103 kg/m3 | Average particle size, microns | Applications | |
| granules | scales | |||||
| 1. Aluminum powder | Al | GOST 5494–71 ISO 1247 ASTM D962 DIN 55923 BS 388 | 2,5–2,7 | 2 | up to 50 | Anti-corrosion paints, coatings for exterior use, for vehicles, dispersion paints, printing inks, electrically conductive coatings, coatings for decorative and lighting purposes |
| 2. Zinc dust | Zn | ISO 3549 ASTM D520-51 DIN 55969 BS 3982 | 7,0–7,1 | 2–9 | 4–10 | Anti-corrosion primers and coatings |
| 3. Powders: | ||||||
| copper | Cu | ASTM D964-48T | 7,6–8,0 | 0,1–20 | 8–10 | Decorative coatings, printing inks, antifouling coatings |
| bronze (Zn - 30%) | Cu, Zn | ASTM D267-41 | ||||
| 4. Stainless steel powders | Fe, Cr, Ni | 7,6 | 13–40 | Anti-corrosion paints | ||
Table 14.98
Pearlescent pigments
Notes
:
1. When producing pearlescent pigments, oxides of tin, zirconium, chromium and other metals are also used to create a layer of metal oxide on the surface of mica.
Iron hydroxides, iron glaze and soot are also used to apply mica to the surface. 2. Relationship between linear dimensions: 1 µm = 103 nm. Targeted pigments
Heat-sensitive pigments
Heat-sensitive pigments include pigments and compounds that change color at a certain temperature. They are divided into two groups. The first group includes substances that, when cooled, acquire their original color - these are reversible pigments; they serve as indicators only for low temperatures within 100 ° C. Irreversible heat-sensitive pigments, when heated to a certain temperature, transform into oxides colored in a color different from the color of the original compounds. In table 14.99 shows the characteristics of heat-sensitive pigments.
Table 14.99
Characteristics of heat-sensitive pigments
| Connections | Color change temperature, °C | Original color | Color after exposure to temperature |
| Reversible | |||
| CoCl2 2C6H12N4 10H2O | 35 | Pink | Blue |
| CoBr2 2C6H12N4 10H2O | 40 | Pink | Blue |
| HgI2 2AgI | 45 | Dark yellow | Dark brown |
| CoI2 2C6H12N4 10H2O | 50 | Pink | Green |
| CoSO4 2C6H12N4 9HO | 60 | Pink | Violet |
| NiCl2 2C6H12N4 10H2O | 60 | Light green | Yellow |
| NiBr2 2C6H12N4 10H2O | 60 | Light green | Blue |
| HgI2 2CuI | 65 | Carmine red | Chocolate |
| Co(NO3)2 2C6H12N4 10H2O | 75 | Pink | Purple |
| Irreversible | |||
| NiNH4PO4 6H2O | 120 | Light green | Gray-green |
| Co3(PO4)2 8H2O | 140 | Pink | Blue |
| CoNH4PO4 H2O | 140 | Purple | Dark blue |
| Pb(OH)2 + 4.5% H2O | 145 | White | Yellow |
| NH4VO3 | 150 | White | Brown |
| (NH4)3PO4 12MoO3 | 160 | Yellow | Black |
| (NH 4)2V2O7 | 200 | Yellow | Grey |
| Cd(OH)2 | 200 | White | Yellow |
| 7CuO 2SO3 6H2O | 220 | Blue | Brown |
| CoCO3 nCo (OH)2 | 250 | Pink | Black |
| FeOOH | 280 | Yellow | Red-brown |
| 2PbCO3 Pb(OH)2 | 285 | White | Yellow |
| PbCO3 | 290 | White | Yellow |
| CdCO3 | 310 | White | Brown |
| CoCl2 | 400 | Pink | Dark brown |
| CuCO3 | 400 | Light green | Dark brown |
| NH4MnP2O7 | 400 | Violet | White |
| Cu(OH)2 Cu3(PO4)2 | 650 | Grey | Green |
Pigments for lighting coatings
Light pigments consist of a base - a luminous substance, into the crystal lattice of which an activator metal is introduced in an amount of 1–10 g/mol of base. The particle size of pigments is 1–5 microns. They are used in television equipment, oscilloscopes, X-ray equipment, radar installations, special-purpose lamps, etc. Table. 14.100 shows the characteristics of pigments for coatings for lighting purposes.
Table 14.100
Pigments-light compounds
| Activator ion | Basis for light composition | Base formula | Wavelength of emitted light, nm | Color |
| Mn2+ | Zinc orthosilicate | ZnSiO3 | 525 | Green |
| Mn2+/Sb3+ | Calcium halogen phosphate | Ca5(PO4)3(Cl, F) | 480,580 | Blue or yellow-orange |
| Mn4+ | Magnesium fluoro-germanate | Mg2GeO4 1.5MgO 0.5MgF | 710 | Red |
| Sn2+ | Magnesium strontium orthophosphate | (Sr, Mg)3(PO4)2 | 630 | Rose red |
| Ce3+ | Yttrium aluminate | Y3Al5O12 | 550 | Yellow |
| Eu2+ | Fluorine-bromo-barium chloride | BaF(Cl, Br) | 440 | Blue |
| Eu3+ | Yttrium oxide | Y2O3 | 625 | Red |
| Tb3+ | Yttrium oxide-sulfide | Y2O2S | 525, 440 | Green, blue |
| Ag+/Cl– | Zinc sulfide | ZnS | 525 | Green |
| Cu+/Cl– | Zinc sulfide | ZnS | 525 | Green |
| Zn2+ | Zinc oxide | ZnO | 505 | Green |
Magnetic pigments
Magnetic pigments (or magnetic powders) have needle-shaped particles, particle size - 0.03–0.1 microns, length to diameter ratio (5: 1) / (10: 1). They are used as storage media material in modern audio, video and computer media. In table 14.101 shows the main properties of magnetic pigments.
Table 14.101
Magnetic pigments
| Magnetic pigment | Coercive force, E* | Residual magnetic induction, T | Squareness factor |
| γ-Fe2O3 | 275–330 | 0,12–0,15 | 0,74–0,76 |
| γ-Fe2O3 modified with cobalt (Co) | 350–1000 | 1,0 | 0,75–0,90 |
| CrO2 modified with antimony (Sb) | 420–500 | 0,18–0,23 | 0,85–0,93 |
| Fe metal | 400 | 0,3 | 0,80–0,88 |
Special Purpose Pigments
Table 14.102
Pigments for anti-friction coatings - solid lubricants
| Properties | Solid lubricants | ||
| Graphite, C | Molybdenum disulfide, MoS2 | Bornitride, BN | |
| Color | Black | Gray-black | White |
| Hardness | 0,89–1,26 | 1,26–1,43 | 2,0 |
| Density, 103kg/m3 | 2,1–2,3 | 4,80 | 2,29 |
| Crystal structure | Hexagonal (layered lattice) | Hexagonal | Hexagonal |
| Temperature limit, °C | 600 | 450 | 300–750 |
| Oxidation products | CO, CO2 | MoO3, SO2 | |
Pigments for fire retardant paints
:
Aluminum hydroxide, natural magnesium silicate - asbestos, antimony trioxide, kaolin, lithopone.
Pigments for heat-resistant coatings
:
Aerosil, mica, aluminum hydroxide, titanium dioxide, chromium oxide, red iron oxide pigments, chalk, talc, black heat-resistant pigment and other heat-resistant pigments.
Pigments for antifouling coatings:
Copper powder, zinc dust, copper-nickel powder, copper(I) oxide Cu2O, copper(I) thiocyanate CuCNS, sulfate lead white.
Table 14.103
Areas of application of white pigments and fillers
| Name of pigment, filler | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
| White pigments | |||||||||||||||
| 1. Titanium dioxide: anatase modification | + | + | + | – | – | – | + | + | + | + | + | + | + | + | + |
| rutile modification | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 2. Zinc oxide | + | + | – | + | + | – | + | + | – | – | – | – | – | + | + |
| 3. Zinc sulfide | o | + | + | o | – | o | – | – | – | + | – | – | – | – | + |
| 4. Lithopone | + | + | o | + | – | o | + | – | – | + | – | + | – | – | + |
| 5. Lead white (carbonate) | + | + | – | + | + | – | – | + | – | – | – | – | – | – | + |
| 6. Lead white (sulfate) | + | + | – | + | + | + | – | – | – | – | – | – | – | – | – |
| Fillers | |||||||||||||||
| 1. Natural calcium carbonate | o | o | o | o | o | – | + | – | – | o | – | + | – | – | – |
| 2. Precipitated calcium carbonate | + | + | o | – | + | + | + | + | + | + | – | + | – | – | + |
| 3. Dolomite | + | – | – | + | + | + | + | – | – | + | – | + | – | – | – |
| 4. Natural barium sulfate | + | – | o | o | o | o | – | – | – | + | – | + | – | – | + |
| 5. Synthetic barium sulfate | + | + | + | + | + | + | + | + | + | + | + | – | + | – | + |
| 6. Diatomite | + | + | – | + | + | – | + | + | – | + | – | – | + | – | – |
| 7. Quartz | + | – | – | – | – | – | – | + | – | – | – | – | + | – | + |
| 8. Silica gel | + | o | – | + | + | + | – | – | – | – | – | – | + | – | + |
| 9. Aerosil | + | + | – | + | + | + | – | + | – | + | – | – | – | – | + |
| 10. Kaolin | + | + | + | o | + | + | + | + | – | + | – | + | + | + | + |
| 11. Bentonite | o | – | – | + | – | o | + | + | – | – | – | – | – | – | – |
| 12. Talc | + | + | – | + | + | – | + | – | – | – | – | – | + | – | – |
| 13. Wollastonite | + | + | + | + | + | + | + | – | – | + | – | – | – | + | – |
| 14. Synthetic calcium silicate | + | + | + | + | + | + | + | – | – | – | – | – | – | – | – |
| 15. Mica: muscovite | + | + | – | – | – | – | + | – | – | + | – | + | + | – | + |
| 16. Mica: flagopite | + | + | – | – | – | – | + | – | – | + | – | + | + | – | + |
| 17. Aluminum hydroxide | + | + | + | + | + | o | + | + | – | + | – | + | + | – | + |
Notes : 1. The digital columns in the table correspond to the following areas of use: 1 - in soils, 2 - in topcoat paints, 3 - in printing inks, 4 - in anti-corrosion paints, 5 - in paints for exterior use, 6 - in paints for transport means, 7 - in dispersion paints, 8 - in artistic paints, 9 - in paint materials for painting lime, cement and concrete, 10 - in paint materials for painting plastics in bulk, 11 - in paintwork materials for painting chemical fibers in bulk, 12 - in LKM for building materials, 13 - in LKM for painting paper, 14 - in LKM for painting ceramic products, 15 - in LKM for painting linoleum, artificial leather, rubber products. 2. “+” means that the product is used or can be used in this area, “–” means it is not used, “o” means it has limited use.
Table 14.104
Areas of application of natural pigments
| Name of pigment, filler | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
| 1. Yellow ocher | + | + | + | + | + | o | + | + | + | – | – | + | + | – | + |
| 2. Natural sienna | + | – | o | o | + | o | – | + | + | – | – | + | + | – | + |
| 3. Burnt umber | + | + | + | + | + | + | + | + | – | – | – | – | – | – | – |
| 4. Iron minium | + | + | + | + | + | + | + | – | + | – | – | + | – | – | + |
| 5. Red ocher | + | + | + | + | + | + | + | + | + | + | – | + | – | + | + |
| 6. Mummy | + | + | + | + | + | + | + | + | + | + | – | + | – | + | + |
| 7. Red ocher obtained by calcination of yellow ocher | – | + | + | – | + | – | + | + | – | – | – | – | + | – | + |
| 8. Mars brown light and dark | + | + | + | + | + | + | + | + | + | – | – | + | – | – | + |
| 9. Iron mica | – | + | – | + | + | – | – | – | – | – | – | – | – | – | – |
| 10. Manganese brown | + | + | + | + | + | – | – | + | + | – | – | + | – | – | + |
| 11. Manganese black | – | + | – | – | – | – | – | + | + | – | – | + | – | – | + |
| 12. Black clayey | – | + | – | – | – | – | – | + | + | – | – | + | – | – | + |
| 13. Shungite | – | + | – | – | – | – | – | + | + | – | – | + | – | – | – |
| 14. Glauconite | – | – | – | – | – | – | – | + | + | – | – | + | – | – | – |
Notes
1.
Feodosia brown, Kassel brown (vandik brown), volkonskoite and vivianite are used exclusively for artistic paints and restoration work. 2. The digital columns in the table correspond to the following areas of use: 1 - in soils, 2 - in topcoat paints, 3 - in printing inks, 4 - in anti-corrosion paints, 5 - in paints for exterior use, 6 - in paints for vehicles, 7 - in dispersion paints, 8 - in artistic paints, 9 - in paint and varnish materials for painting lime, cement and concrete, 10 - in paint and varnish materials for painting plastics in bulk, 11 - in paint and varnish materials for painting chemical fibers in bulk, 12 - in paint and varnish materials for building materials, 13 - in paint and varnish materials for painting paper, 14 - in paint and varnish materials for painting ceramic products, 15 - in paint and varnish materials for painting linoleum, artificial leather, rubber products. 3. “+” means that the product is used or can be used in this area, “–” means it is not used, “o” means it has limited use.
Table 14.105
Areas of application of synthetic pigments
| Pigment name | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
| 1. Yellow iron oxide pigment | + | + | + | + | + | + | + | + | + | + | – | + | + | – | – |
| 2. Mars yellow transparent | – | – | – | – | – | + | – | + | – | – | – | – | – | – | – |
| 3. Cadmium yellow pigments | – | + | + | – | o | + | + | + | – | + | + | – | – | + | + |
| 4. Yellow cadmopons | – | + | + | – | o | + | + | + | – | + | + | – | – | + | + |
| 5. Yellow lead crown | + | + | + | + | + | + | + | + | – | + | + | – | + | – | + |
| 6. Orange lead crown | + | + | – | + | + | + | – | – | – | – | – | – | – | – | – |
| 7. Zinc crown | + | – | – | + | – | – | – | + | – | – | – | – | – | – | – |
| 8. Strontium crown | + | + | – | + | – | – | – | + | – | – | – | + | – | – | – |
| 9. Nickel titanate | – | + | + | – | + | + | + | – | – | + | – | + | – | + | + |
| 10. Yellow iron-zinc pigment | – | + | – | – | + | + | + | – | – | + | – | – | – | + | + |
| 11. Red cadmium pigments | – | + | + | – | + | + | + | + | – | + | + | – | + | + | – |
| 12. Red cadmopons | – | + | + | – | + | + | + | + | – | + | + | – | + | + | – |
| 13. Mercury cinnabar | – | – | – | – | – | – | – | + | – | – | – | – | – | – | – |
| 14. Mercury-cadmium pigments | – | + | + | – | + | + | + | + | – | + | + | – | + | + | – |
| 15. Lead-molybdate crown | + | + | + | + | + | + | – | – | – | + | + | – | + | – | + |
| 16. Red iron oxide pigments | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 17. Brown iron oxide pigment | + | + | + | + | + | + | + | + | + | + | + | + | + | – | + |
| 18. Brown iron oxide pigment mixed | + | + | + | + | + | + | + | + | – | – | – | + | + | – | + |
| 19. Mars orange transparent | – | – | – | – | – | + | – | + | – | – | – | – | – | – | – |
| 20. Mars brown dark transparent | – | – | – | – | – | – | – | + | – | – | – | – | – | – | – |
| 21 Chromium titanate | – | + | + | – | + | + | + | – | – | – | + | + | – | – | + |
| 22. Brown oxide pigment | o | + | – | o | + | + | + | – | + | + | – | + | – | + | + |
| 23. Chromium oxide | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 24. Emerald green, Guinier green | – | – | – | – | – | – | – | + | – | – | – | – | – | – | – |
| 25. Green cobalt pigments | – | + | – | – | – | – | – | + | – | – | – | – | – | – | – |
| 26. Green cobalt titanate | – | + | – | – | + | – | – | + | – | + | – | – | – | + | – |
| 27. Ultramarine | + | + | + | + | + | + | + | + | + | + | – | + | + | – | + |
| 28. Cobalt Blue | – | + | + | – | + | + | + | + | – | + | – | – | – | + | – |
| 29. Blue cobalt-zinc silicate | – | – | – | – | – | – | – | + | – | – | – | – | – | + | – |
| 30. Chrome-cobalt green-blue | – | – | – | – | – | – | – | + | – | – | – | – | – | + | – |
| 31. Cerulean | – | – | – | – | – | – | – | + | – | – | – | – | – | + | – |
| 32. Manganese blue | – | – | – | – | – | – | – | + | – | – | – | – | – | – | – |
| 33. Iron blue, milori | o | + | + | o | + | + | – | + | – | – | – | + | + | – | + |
| 34. Cobalt violet dark | – | – | – | – | – | – | – | + | – | – | – | – | – | – | – |
| 35. Cobalt violet light | – | – | – | – | – | – | – | + | – | – | – | – | – | – | – |
| 36. Manganese violet | – | – | – | – | + | – | – | + | – | – | – | – | – | – | – |
| 37. Black iron oxide pigment | + | + | – | + | + | + | + | + | + | + | – | + | – | – | + |
| 38. Black heat-resistant pigment based on metal oxides | + | + | + | – | + | + | + | – | – | + | – | + | – | + | + |
| 39. Burnt bone | + | + | + | + | + | + | + | + | – | – | – | – | – | – | – |
| 40. Grape and peach black | – | – | – | – | – | – | – | + | – | – | – | – | – | – | – |
| 41. Channel gas soot | o | + | – | o | + | + | + | + | + | + | + | + | + | – | + |
| 42. Stove soot | – | + | – | – | + | + | – | – | + | O | – | + | – | + | + |
| 43. Lamp soot | + | + | + | + | + | + | + | + | – | + | + | – | + | – | – |
| 44. Thermal soot | o | – | – | – | o | – | – | – | + | – | – | + | – | – | + |
Notes : 1. The digital columns in the table correspond to the following areas of use: 1 - in soils, 2 - in topcoat paints, 3 - in printing inks, 4 - in anti-corrosion paints, 5 - in paints for exterior use, 6 - in paints for transport means, 7 - in dispersion paints, 8 - in artistic paints, 9 - in paint materials for painting lime, cement and concrete, 10 - in paint materials for painting plastics in bulk, 11 - in paintwork materials for painting chemical fibers in bulk, 12 - in LKM for building materials, 13 - in LKM for painting paper, 14 - in LKM for painting ceramic products, 15 - in LKM for painting linoleum, artificial leather, rubber products. 2. “+” means that the product is used or can be used in this area, “–” means it is not used, “o” means it has limited use.
Mohs hardness of steel
The mineralogical hardness scale, the Mohs hardness scale,
is called both
a set of standard minerals
for determining the relative hardness by scratching the test object with a standard, and the actual ten-point
scale of relative hardness of minerals
.
as the standards of the Mohs mineralogical hardness scale
, which are arranged on the scale in order of increasing hardness: talc, gypsum, calcite, fluorite, apatite, orthoclase (feldspar), quartz, topaz, corundum, diamond.
The Mohs hardness scale has been proposed as a relative hardness scale
in 1811 by the German scientist Friedrich Mohs (F. Mohs, F. Mohs). Despite the fact that the development dates back to the beginning of the 19th century, this conventional hardness scale is widely used to this day. Today it is possible to purchase a set of standard minerals both for the educational process and for the jewelry industry.
Mohs hardness scale
used for rapid comparative diagnostics of minerals.
Moreover, if a standard of the hardness scale, having a hardness of 5, scratches the sample under study, and the latter leaves a mark on the surface of the standard with a hardness of 4, then the intermediate hardness of the mineral is equal to 4.5 (4½) on the Mohs scale. At the same time, it must be borne in mind that the Mohs hardness scale is not a linear scale. The number on the hardness scale only indicates the order of the hardness distribution, but does not have any quantitative meaning. In no case does it follow from the hardness scale that, for example, diamond (10) is twice as hard as apatite (5). Absolute hardness values (sometimes called truly relative
) present a completely different picture. In addition, it must be taken into account that the hardness of some minerals in different directions can vary greatly due to the crystalline structure. The hardness of a metal has more stable absolute values, but in practice we rarely deal with pure metal, and the hardness of an alloy generally depends on all its components.
The Mohs hardness scale in the technical literature is usually depicted in the form of a comparative table. For clarity, it is sometimes accompanied by photographs of minerals - standards of the hardness scale.
.
Mohs hardness scale
It is also convenient because [3] you can practically use such “improvised means” as a fingernail (Mohs hardness 2½), a cent or any other US coin (hardness slightly less than 3), a knife (Mohs hardness 5½), glass ( 5½), high-quality steel file (hardness 6½), sandpaper using synthetic corundum (has a Mohs hardness of 9), fine wood sandpaper “garnet paper” (hardness 7½).
| 1 - Talc | 2 - Plaster | 3 - Calcite | 4 - Fluorite | 5 - Apatite |
| 6 - Orthoclase | 7 – Quartz | 8 – Topaz | 9 – Corundum | 10 – Diamond |
Review author: Kornienko A.E. (ICM)
Lit.:
- Ivanov V.N. Dictionary-reference book for foundry production. – M.: Mechanical Engineering, 1990. – 384 p.: ill. ISBN 5-217-00241-1
- Great Soviet Encyclopedia - M., 1969-1978.
- The Mohs Mineral Hardness Scale. By Andrew Alden // About.com: Geology. [Electronic resource], 2010 - Access mode: https://geology.about.com/od/scales/a/mohsscale.htm, free. — Cap. from the screen.
- Mohs Hardness Scale // ScienceViews.com - an image filled educational website featuring animals, herbs, petroglyphs, pictographs, rock art, reptiles, bugs, birds, mammals, nature, parks, and outdoor online science for your enjoyment, research and learning. [Electronic resource], 2003-2008 - Access mode: https://scienceviews.com/, free. — Cap. from the screen.
Competition “Me and my profession: metallurgist, foundry technologist.” Find out, participate >>>
—>
The essence of the method and the searches of scientists
The principle of the method turned out to be simple: Mohs took plaster and could not scratch any other stone with it. He conditionally defined its hardness as 1.
He arranged the following . The last stone, number 10, was a diamond that could not be scratched by any other nugget.
For example, hardness is 7 if neither the gem under study nor the quartz damage each other.
Selected standards
The scientist chose 10 minerals , the correspondence to one of which is called hardness on the Mohs scale.
The table lists minerals - standards of hardness.
| Mineral | Properties | |
| 1 | Talc | It is easily scratched even with a fingernail and will not harm any mineral. The hardness of graphite is approximately the same, which is why a simple pencil is often used as a material at hand when checking. |
| 2 | Gypsum | It is damaged by the fingernail and leaves scratches on the talcum powder. |
| 3 | Calcite | Scratches the previous standard. |
| 4 | Fluorite | Damages the previous standard and is scratched by a knife. |
| 5 | Apatite | Glass hardness on the Mohs scale is 5.5. Consequently, a gem can also be damaged by glass by applying great force. |
| 6 | Orthoclase | It scratches the glass when pressed hard and is damaged by a steel file. |
| 7 | Quartz | Stronger than glass and softer than diamond. |
| 8 | Topaz | Durable mineral, scratches quartz, glass. Grinds with diamond-coated tools. |
| 9 | Corundum | Second only to diamond. |
| 10 | Diamond | Maximum hardness. |
Quantitative values
The Mohs scale is a reflection of the relative strength of stones. That is, if talc is in first place, and diamond is in 10th place, this does not mean that the difference between their hardness is a multiple of only 10.
In fact, diamond is 1500 times harder than talc. The absolute hardness of all nuggets is measured using special devices - sclerometers.
This is what the expanded table of minerals with absolute indicators looks like.
| Relative value | Mineral | Absolute value |
| 1 | Talc | 1 |
| 2 | Gypsum | 3 |
| 3 | Calcite | 9 |
| 4 | Fluorite | 21 |
| 5 | Apatite | 48 |
| 6 | Orthoclase | 72 |
| 7 | Quartz | 100 |
| 8 | Topaz | 200 |
| 9 | Corundum | 400 |
| 10 | Diamond | 1500 |
Apparent disadvantages
Despite the conventionality of the indicators, all attempts to refine the scale have not received recognition . It seemed wrong to scientists to take calcite as a standard due to the fact that its hardness varies. But the mineral galena, similar to it, also did not become ideal in this indicator.
Therefore, the only worldwide recognized system for classifying minerals by hardness remains the Mohs scale.
Scientific research
There are other classifications of minerals by hardness: Knoop, Brinell, Rockwell or Vickers . They are based on the stone’s resistance not to scratching, but to indentation.
The measurement is made using a special device that presses on the mineral with a given force. Using a formula, the strength is calculated based on the force and the corresponding depth of the hole. The devices differ from each other , so the numbers are different, making it impossible to compare the values of different methods.
Other scientists have come up with more technically complex calculation methods. Despite the accuracy of the indicators, most people understand and are more accustomed to comparing the hardness of stones on the Mohs scale .
Hardness of metals
Mechanical engineering parts and mechanisms, as well as tools intended for their processing, have a set of mechanical characteristics. Hardness plays a significant role among the characteristics. The hardness of metals clearly shows:
- wear resistance of metal;
- possibility of processing by cutting, grinding;
- resistance to local pressure;
- ability to cut other materials and others.
In practice, it has been proven that most of the mechanical properties of metals directly depend on their hardness.
Hardness concept
The hardness of a material is its resistance to destruction when a harder material is introduced into the outer layer. In other words, the ability to resist deforming forces (elastic or plastic deformation).
The hardness of metals is determined by introducing a solid body called an indenter into a sample. The role of the indenter is performed by: a metal ball of high hardness; diamond cone or pyramid.
After exposure to the indenter, an imprint remains on the surface of the test sample or part, the size of which determines the hardness. In practice, kinematic, dynamic, and static methods of measuring hardness are used.
The kinematic method is based on the compilation of a diagram based on continuously recorded readings that change as the tool is pressed into the sample. Here the kinematics of the entire process is traced, and not just the final result.
The dynamic method is as follows. The measuring tool acts on the part. The reverse reaction allows you to calculate the expended kinetic energy. This method allows you to test the hardness of not only the surface, but also a certain volume of metal.
Static methods are non-destructive methods that allow you to determine the properties of metals. The methods are based on smooth indentation and subsequent holding for some time. The parameters are regulated by methods and standards.
The applied load can be applied:
- pressing;
- scratching;
- cutting;
- rebound
Machine-building enterprises currently use the Brinell, Rockwell, Vickers methods, as well as the microhardness method, to determine the hardness of materials.
Based on the tests carried out, a table is compiled indicating the materials, the applied loads and the results obtained.
Hardness Units
Each method of measuring the resistance of a metal to plastic deformation has its own methodology, as well as units of measurement.
The hardness of soft metals is measured using the Brinell method. Non-ferrous metals (copper, aluminum, magnesium, lead, tin) and alloys based on them, cast iron (except for white) and annealed steel are subjected to this method.
Brinell hardness is determined by indentation of a hardened, polished ball made of ShKh15 ball bearing steel. The circumference of the ball depends on the material being tested. For hard materials - all types of steel and cast iron - 10 mm, for softer materials - 1 - 2 - 2.5 - 5 mm. Required load applied to the ball:
- iron alloys – 30 kgf/mm2;
- copper and nickel – 10 kgf/mm2;
- aluminum and magnesium – 5 kgf/mm2.
The unit of hardness measurement is a numerical value followed by a numerical index HB. For example, 200 NV.
Rockwell hardness is determined by the difference in applied loads to the part. First, a preliminary load is applied, and then a general load, at which the indenter is introduced into the sample and held.
A pyramid (cone) of diamond or a ball of tungsten carbide (hardened steel) is introduced into the test sample. After removing the load, the depth of the indentation is measured.
The unit of measurement for hardness is conventional units. It is generally accepted that one is the amount of axial displacement of the cone, equal to 2 μm. The hardness designation is marked with three letters HR (A, B, C) and a numerical value. The third letter in the marking indicates the scale.
The technique reflects the type of indenter and the load applied to it.
| Scale type | Tool | Applied load, kgf |
| A | Diamond cone with 120° apex angle | 50-60 |
| IN | 1/16" ball | 90-100 |
| WITH | Diamond cone with 120° apex angle | 140-150 |
Basically, measurement scales A and C are used. For example, the hardness of steel is HRC 26...32, HRB 25...29, HRA 70...75.
Products of small thickness or parts with a thin, hard surface layer are measured by Vickers hardness. The blade used is a regular tetrahedral pyramid with an apex angle of 136°. The display of hardness values is as follows: 220 HV.
Hardness measurement using the Shore method is carried out by measuring the rebound height of a fallen striker. Indicated by numbers and letters, for example, 90 HSD.
Microhardness is determined when it is necessary to obtain the values of small parts, thin coatings, or individual alloy structures. The measurement is made by measuring the imprint of a tip of a certain shape. The value notation looks like this:
0.196 — tip load, N;
2800 – numerical value of hardness, N/mm 2.
Learn to determine the hardness of a stone yourself
Greetings, my dears! In the last article we learned everything about rock crystal
and how difficult it is to process, so today it is naturally worthwhile to understand what this mineral hardness actually is, how to determine it and what gradations exist. Well, don’t forget to subscribe to the channel and, of course, like it - because this determines whether the channel will continue to live.
Let's remember the general principles for determining the naturalness of stones. As we already discussed
, one of the main materials for counterfeiting is glass, and to immediately understand that it is glass and not stone, you just need to check the hardness. Do you remember how to do this? That's right, try to scratch, for example, a mirror with the test stone. It will leave a scratch, which means it is harder than glass, therefore, most likely, what you have is not a fake. He won’t leave - there is reason to doubt. Of course, this method is not universal, because there are dozens of jewelry minerals in the world that are softer than glass, for example, fluorite or amber, but in most cases the scratch test still works. So, in 1811, the German mineralogist Friedrich Mohs developed a stone hardness scale, which jewelers and gemologists still use today. This scale, of course, is very arbitrary, but of all the existing ones, it is the most convenient and universal. The fact is that Mohs came up with the idea of using 10 minerals as divisions of this scale, the hardness of which increases from a conventional unit to ten. The stones were chosen at random; most likely, he simply used the most famous ones. Of course, no one doubts that the standard of hardness was diamond - any schoolchild knows that this is the hardest mineral on earth. So its indicator is 10. Talc was taken as one, which, although it has the appearance of a crystal, is easily scratched even with a fingernail. All other stones are located between these two points.
So, the Mohs hardness scale
(reference mineral in bold).
2. Gypsum
. Also mica, halite, chlorite.
3. Calcite
. Also biotite, silver and gold. You can no longer scratch it with a fingernail, but even copper can scratch it easily.
4. Fluorite
. Also sphatelite and dolomite. Can be scratched with a knife or a piece of glass.
Source
Hardness of base metals and alloys
The hardness value is measured on finished parts sent for assembly. Control is carried out for compliance with the drawing and technological process. Tables of hardness values have already been compiled for all basic materials, both in the initial state and after heat treatment.
Non-ferrous metals
The Brinell hardness of copper is 35 HB, the values of brass are 42-60 HB units, depending on its brand. For aluminum, the hardness is in the range of 15-20 HB, and for duralumin it is already 70 HB.
Black metals
The Rockwell hardness of cast iron SCH20 HRC 22, which corresponds to 220 HB. Steel: tool – 640-700 HB, stainless steel – 250 HB.
To convert from one measurement system to another, tables are used. The values in them are not true, because they are derived imperially. Not the full volume is presented in the table.
| HB | H.V. | H.R.C. | HRA | HSD |
| 228 | 240 | 20 | 60.7 | 36 |
| 260 | 275 | 24 | 62.5 | 40 |
| 280 | 295 | 29 | 65 | 44 |
| 320 | 340 | 34.5 | 67.5 | 49 |
| 360 | 380 | 39 | 70 | 54 |
| 415 | 440 | 44.5 | 73 | 61 |
| 450 | 480 | 47 | 74.5 | 64 |
| 480 | 520 | 50 | 76 | 68 |
| 500 | 540 | 52 | 77 | 73 |
| 535 | 580 | 54 | 78 | 78 |
Hardness values, even if produced by the same method, depend on the applied load. The lower the load, the higher the readings.