Table salt is a substance widely used in the food industry, medicine, animal husbandry, cosmetology, etc. since ancient times. This white crystalline powder is obtained by different methods. This could be, for example, evaporation of sea water, mining in quarries, collecting from the bottom of lakes. But in any case, the final product always has the same physical characteristics. For example, what is the hardness of salt on the Mohs scale? We’ll talk about that later in the article. We will also look at what other characteristics this very popular product has.
What is the Mohs scale
You might be interested in: What are phenomena? The most beautiful and terrible natural phenomena
One of the distinguishing features of many substances on the planet is the degree of hardness. This parameter is determined according to a special scheme called the Mohs scale. To facilitate the task of comparing the hardness of different substances, 10 standard elements are included in this system. The hardness of these substances is checked simply by scratching.
The hardest mineral on the planet, diamond, ranks first on the Mohs scale. This gemstone is known to not be scratched even by a strong steel knife. The hardness of a diamond on the Mohs scale is therefore 10. In second place in this scheme are corundums - rubies and sapphires. Their hardness is 9. The softest reference substances on the Mohs scale are talc and chalk. Their hardness in this scheme is defined as 1.
Mohs hardness scale
The Mohs scale (mineralogical hardness scale) is a qualitative ordinal scale that characterizes the scratch resistance of various minerals. Used to determine the relative hardness of mineral samples.
Based on the ability of a harder material to scratch a softer material.
The scale contains 10 minerals as reference minerals, ranking them in order of increasing hardness from very soft (talc) to very hard (diamond).
All of the minerals in the table, except diamond, are relatively common and easy or inexpensive to obtain.
- — Talc
- — Plaster
- — Calcite
- — Fluorite
- — Apatite
- — Orthoclase
- — Quartz
- — Topaz
- — Corundum
- — Diamond
If a mineral scratches a standard, then its hardness is higher; if it is scratched by a standard, it means lower.
The Mohs scale was created in 1812 and named after its inventor, German geologist and mineralogist Friedrich Mohs. Since then, many different methods for determining hardness have been invented: the Brinell, Knoop, Rockwell, Shore, Vickers method.
Mohs hardness is a relative integer comparison of scratch resistance.
Other hardness measurement methods rely on indentation resistance. For testing, an “Indenter” is used, which is pressed into the test sample with a carefully measured force. The size or depth of the notch in the specimen and the magnitude of the force are then used to calculate the hardness value. Because each of these tests uses different apparatus and different calculations, they cannot be directly compared to each other.
The Mohs scale has become widespread because... The hardness test method is easy to perform, inexpensive and quickly understood by people.
Despite its lack of accuracy, the scale is useful for field geologists who use it for rough identification of minerals when examining easily identifiable samples or when more complex tests are not available.
Some use readily available items for a quick test. For example, a geologist may have a pocket knife that can be used to determine whether a sample is harder or softer than a Mohs value of 5-6.5.
- 1 - Pencil
- 2 - Table salt
- 2-2.5 - Can be scratched with a fingernail
- 2.5-3 - Gold, silver
- 3 – Copper coin
- 4-4.5 - Nail
- 4-5 - Iron
- 5 - Glass
- 5-6.5 - Knife blade
- 6.5 - Steel file
- 7 - Easily scratches glass
- 7+ — Hardened steel file
- 8 - Sandpaper, mineral scratches glass very easily
- 9 – Mineral cuts glass
- 10 - Used as a glass cutter
Below is an extended table of substances, minerals, and precious stones:
| Substance or mineral | Mohs hardness |
| Pyrophyllite, molybdenite | 1-2 |
| Bauxite, coal | 1-3 |
| Limonite | 1-5 |
| Ice, sugar, gallium, strontium, indium, tin, barium, thallium, lead, graphite | 1,5 |
| Gypsum, calcium | 1,5-2 |
| Sulfur | 1,5-2,5 |
| Sylvite, glauconite, cadmium, selenium | 2 |
| Rock salt, cinnabar, chlorite, bismuth, amber | 2-2,5 |
| Muscovite | 2-3 |
| Silver, gold, galena, copper, biotite, mica | 2,5-3 |
| Aluminum, limestone, calcite, boric acid, nitrophoska | 3 |
| Aragonite, witherite, anhydrite | 3-3,5 |
| Pearl, brass, arsenic | 3-4 |
| Serpentine | 3-5 |
| Sphalerite, rhodochrosite, malachite, dolomite, cuprite, chalcopyrite, azurite, barite | 3,5-4 |
| Siderite, pyrrhotite, dolomite | 3,5-4,5 |
| Fluorite, phosphor bronze | 4 |
| Marble | 4-5 |
| Tooth enamel, asbestos, apatite, manganese, zirconium, palladium, obsidian | 5 |
| Titanite, monazite | 5-5,5 |
| Jade, uraninite, ilmenite, enstatite, porcelain stoneware (polished) | 5-6 |
| Magnetite | 5-6,5 |
| Nepheline, augite, arsenopyrite, actinolite, bustamite, cobaltite | 5,5-6 |
| Rhodonite, diopside, opal, red iron ore | 5,5-6,5 |
| Titanium, germanium, niobium, rhodium, uranium | 6 |
| Rutile, pyrite, prehnite, plagioclase, orthoclase, amazonite, andesine, anorthoclase, benitoite, helvite, iridium | 6-6,5 |
| Silicon | 6,5 |
| Jasper | 6,5-7 |
| Agate, zoisite, epidote, cassiterite, pyrolusite | 6-7 |
| Marcasite | 6-7,5 |
| Granite, tanzanite, spodumene, olivine, jadeite, axinite, chrysoprase, jadeite | 6,5-7 |
| Sillimanite, garnet | 6,5-7,5 |
| Quartz, stone pebbles, amethyst, aventurine, forsterite, osmium, silicone, rhenium, vanadium | 7 |
| Tourmaline, cordierite, almandine, boracite, cordierite, danburite | 7-7,5 |
| Zircon, andalusite, euclase, hambergite, sapphirine | 7,5 |
| Emerald, hardened steel, tungsten, spinel, beryl, beryllium, aquamarine, red beryl, ganite, painite | 7,5-8 |
| Topaz, cubic zirconia | 8 |
| Chrysoberyl, alexandrite, choltite | 8,5 |
| Porcelain tiles (unpolished) | 8,5 |
| Corundum, ruby, sapphire, alundum, chrome | 9 |
| Moissanite, boron | 9,5 |
| Carborundum | 9-10 |
| Diamond, carbonado | 10 |
What is salt
You may be interested in: What is reaping? Interpretation and example sentences
The chemical formula of this substance is as follows: NaCl. Table salt is also called sodium chloride or rock salt. When crushed, this food product appears as colorless crystals. The latter may have different sizes. In any case, salt in bulk is white.
The main feature of sodium chloride, as is known, is its characteristic taste. In everyday life and in the food industry, table salt can be added to a variety of products. As scientists have found out, sodium chloride is a substance without which human life is completely impossible.
What is the hardness of salt on the Mohs scale?
You may be interested: A caravanserai is... Meanings, history, interesting facts
In nature, sodium chloride is a very common substance. Therefore, rock salt was, among other things, included as a standard in the Mohs scale. Sodium chloride is located in this scheme in the penultimate ninth place. That is, the hardness of table salt is two. Sodium chloride crystals are known to be fragile and easily dissolve in water. The salt grains look quite hard. However, this impression is largely misleading. In fact, salt crystals are easily scratched even with just a fingernail.
Mohs hardness
Content
- Introduction
- Mohs Hardness Metals
- Minerals
Introduction
The hardness of bodies, especially minerals, is often assessed on a completely arbitrary ten-point scale, which is usually called the Mohs scale
. Ten minerals serve as hardness standards in this scale (Table 1).
These minerals are selected so that each subsequent standard makes a scratch on the surface of the previous one.
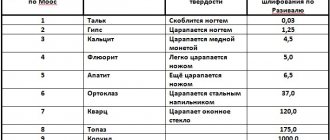
The same technique - making a scratch - also serves to determine the hardness of various other minerals and bodies, since it is always possible to establish that the body under study in terms of hardness either corresponds to one of the standards, or lies in the interval between two neighboring standards. This makes it possible to approximately estimate the hardness of the bodies under study using the corresponding numbers. This definition of hardness is obviously very inaccurate and quite arbitrary, nevertheless, it is often resorted to in practice due to its extreme simplicity. Table 1 - Mohs hardness standards
| Material | Hardness |
| Talc | 1 |
| Gypsum (or rock salt) | 2 |
| Lime spar | 3 |
| Fluorspar | 4 |
| Apatite | 5 |
| Feldspar (orthoglaze) | 6 |
| Quartz | 7 |
| Topaz | 8 |
| Corundum | 9 |
| Diamond | 10 |
Mohs hardness
Table 2 - Mohs hardness
| Material | Hardness |
| Metals | |
| Aluminum | up to 2.9 |
| Bismuth | 2,5 |
| Iron | OK. 4.5 |
| Gold | 2,5 |
| Iridium | 6,5 |
| Cadmium | 2,0 |
| Potassium | 0,5 |
| Cobalt | 5,5 |
| Silicon | 7,0 |
| Lithium | 0,6 |
| Magnesium | 2,0 |
| Manganese | 5,0 |
| Copper | 3,0 |
| Sodium | 0,4 |
| Nickel | 5,8 |
| Tin | 1,8 |
| Palladium | 4,8 |
| Platinum | 4,3 |
| Lead | 1,5 |
| Silver | 2,7 |
| Chromium | 9,0 |
| Zinc | 2,5 |
| Minerals | |
| Azurite | 3,6-4,0 |
| Diamond | 10 |
| Anhydride | 3,0-3,5 |
| Antimonite | 2,0-2,5 |
| Aragonite | 3,5-4,0 |
| Arsenopyrite | 5,5-6,0 |
| Orpiment | 1,5-2,0 |
| Baryte (Heavy spar) | 2,5-3,0 |
| Beryl | 7,5-8,0 |
| Bishofite | 1,5-2,0 |
| Brownite | 6,0-6,5 |
| Brookite | 5,5-6,0 |
| Vanadinite | 2,5-3,0 |
| Bismuthin | 2,0-2,5 |
| Witherite | 3,0-3,7 |
| Wolframite | 4,5-5,5 |
| Wulfenite | 2,5-3,0 |
| Gadolinite | 6,6-7,0 |
| Galena | 2,5-3,0 |
| Halite (Rock salt) | 2,0-2,5 |
| Hematite | 5,5-6,5 |
| Gypsum | 1,5-2,0 |
| Glauberite | 2,5-3,0 |
| Graphite | 1,0-2,0 |
| Huebnerite | 4,5-5,5 |
| Diaspora | 6,5-7,0 |
| Diopside | 5,5-6,0 |
| Dolomite | 3,5-4,0 |
| Ilmenite | 5,0-6,0 |
| Calcite (Limespar) | 3,0 |
| Cassiterite | 6,0-7,0 |
| Quartz | 7,0 |
| Kyanite (Disthene) | 4,5-7,0 |
| Cinnabar | 2,0–2,5 |
| Cobaltine | 5,5-6,0 |
| Corundum | 9,0 |
| Xenotime | 4,0-5,0 |
| Limonite (Brown iron ore) | 5,0-5,5 |
| Magnesite | 3,5-4,5 |
| Magnetite | 5,5-6,5 |
| Malachite | 3,5-4,0 |
| Molybdenite | 1,0-1,5 |
| Monazite | 5,0-5,5 |
| Olivine | 6,5-7,0 |
| Opal | 5,5-6,5 |
| Petzit | 2,5-3,0 |
| Pyrite | 6,0-6,5 |
| Pyrrhotite | 3,5-4,5 |
| Realgar | 1,5-2,0 |
| Rutile | 6,0-6,5 |
| Sulfur | 2,0-2,5 |
| Siderite | 3,5-4,5 |
| Silvit | 2,0-2,5 |
| Mica | 2,0-3,0 |
| Sperrylite | 6,0-7,0 |
| Spodumene | 6,5-7,0 |
| Sphalerite | 3,5-4,0 |
| Sphene (Titanit) | 5,0-5,5 |
| Thorianite | 6,5 |
| Thorite | 4,5-5,0 |
| Thorolite | 5,5-6,0 |
| Tremolite | 5,5-6,0 |
| Tourmalite | 7,0-8,0 |
| Uraninite | 5,0-6,0 |
| Phenaktite | 7,5-8,0 |
| Fluorite | 4,0 |
| Chalcozine | 2,5-3,0 |
| Chalcopyrite | 3,5-4,0 |
| Chrysoberyl | 8,5 |
| Chromite | 5,5-7,5 |
| Cerussite | 3,0-3,5 |
| Zircon | 7,5-8,0 |
| Sheelit | 4,5-5,0 |
| Stoltsit | 2,8-3,0 |
| Spinel | 8,0 |
| Enstatite | 5,0-6,0 |
Literature
- Brief physical and technical reference book. T.1 / Ed. ed. K.P. Yakovleva. M.: FISMATGIZ. 1960. – 446 p.
Linear hardness
Thus, as we found out, NaCl occupies the penultimate place on the Mohs hardness scale. The linear hardness of minerals is also very easy to determine using this scheme. Of course, this characteristic is also known for standard sodium chloride.
The relative indicator for salt, as we found out, is 2. What is the absolute hardness of salt on the Mohs hardness scale? For NaCl this figure is 3.
Hardness Units
Each method of measuring the resistance of a metal to plastic deformation has its own methodology, as well as units of measurement.
The hardness of soft metals is measured using the Brinell method. Non-ferrous metals (copper, aluminum, magnesium, lead, tin) and alloys based on them, cast iron (except for white) and annealed steel are subjected to this method.
Brinell hardness is determined by indentation of a hardened, polished ball made of ShKh15 ball bearing steel. The circumference of the ball depends on the material being tested. For hard materials - all types of steel and cast iron - 10 mm, for softer materials - 1 - 2 - 2.5 - 5 mm. Required load applied to the ball:
- iron alloys – 30 kgf/mm2;
- copper and nickel – 10 kgf/mm2;
- aluminum and magnesium – 5 kgf/mm2.
The unit of hardness measurement is a numerical value followed by a numerical index HB. For example, 200 NV.
Rockwell hardness is determined by the difference in applied loads to the part. First, a preliminary load is applied, and then a general load, at which the indenter is introduced into the sample and held.
A pyramid (cone) of diamond or a ball of tungsten carbide (hardened steel) is introduced into the test sample. After removing the load, the depth of the indentation is measured.
The unit of measurement for hardness is conventional units. It is generally accepted that one is the amount of axial displacement of the cone, equal to 2 μm. The hardness designation is marked with three letters HR (A, B, C) and a numerical value. The third letter in the marking indicates the scale.
The technique reflects the type of indenter and the load applied to it.
| Scale type | Tool | Applied load, kgf |
| A | Diamond cone with 120° apex angle | 50-60 |
| IN | 1/16" ball | 90-100 |
| WITH | Diamond cone with 120° apex angle | 140-150 |
Basically, measurement scales A and C are used. For example, the hardness of steel is HRC 26...32, HRB 25...29, HRA 70...75.
Products of small thickness or parts with a thin, hard surface layer are measured by Vickers hardness. The blade used is a regular tetrahedral pyramid with an apex angle of 136°. The display of hardness values is as follows: 220 HV.
Hardness measurement using the Shore method is carried out by measuring the rebound height of a fallen striker. Indicated by numbers and letters, for example, 90 HSD.
Microhardness is determined when it is necessary to obtain the values of small parts, thin coatings, or individual alloy structures. The measurement is made by measuring the imprint of a tip of a certain shape. The value notation looks like this:
Read also: Location of views in the drawing, local views, presentation
0.196 — tip load, N;
2800 – numerical value of hardness, N/mm 2.
Minerals with similar hardness
Salt, therefore, is a fairly soft substance. There are many such minerals in nature. For example, gypsum, mica, and chlorite have the same absolute and relative hardness indicators as NaCl. All these substances are easily scratched with a fingernail.
Of course, sugar also has its place on the relative Mohs hardness scale. Salt is used on the scale as one of the reference substances. Sugar, although also a very common food, is not initially listed on the Mohs chart. However, the hardness of this substance, like any other, is, of course, also known. Sugar is slightly softer than salt, but on the Mohs scale its hardness rating is also 2.
Graduation principles from Mohs
A system for comparing the hardness of crystals was invented by the German geologist Friedrich Moos (another version of the sound is Moss). It is a ten-point scale of reference minerals , arranged in order of increasing strength.
When you try to scratch one crystal with another, a chain is built from the softest stone (talc) to the hardest (diamond). The Mohs hardness scale only informs about the comparative strength of the minerals being compared.
If the test stone does not leave a mark on the quartz face, and the standard does not scratch the sample, then it can be stated that the hardness of the crystal being tested is equal to 7 on the Mohs scale table.
| Control mineral | Number according to the identifier | Other stones of the same strength | Scratching object | Cutter hardness value |
| Soapstone | 1 | Graphite | Nail | 2,5 |
| Gypsum (selenite) | 2 | Halite, mica, chlorite | ~ | ~ |
| Calcite (Iceland spar) | 3 | Biotite, silver, gold | Copper coin | 3,5 |
| Fluorite (fluorspar) | 4 | Sphalerite, dolomite | Iron knife, window glass | 5,5 |
| Apatite | 5 | Lapis lazuli, hematite | ~ | ~ |
| Orthoclase (adularia, feldspar) | 6 | Opal, rutile | Steel nail | 6,5 |
| Quartz (agate, jasper, amethyst, rock crystal, aventurine) | 7 | Tourmaline, garnet | Pobedite drill | 8,5 |
| Topaz | 8 | Aquamarine, emerald, spinel | ~ | ~ |
| Corundum (sapphire, ruby) | 9 | Wolfram carbide | Diamond | 10 |
| Diamond | 10 | Elbor | ― | ― |
For ease of use, the reference mineral is fixed to the end of the tube with epoxy glue. Scratch carefully, trying not to damage the sample. They work in the same way with improvised objects. Since the hardness of glass on the Mohs scale is in the middle position (between values 5 and 6), the test begins with scratching its surface.
Other physical characteristics
So, we found out what the hardness of salt is on the Mohs hardness scale. But what other properties does this substance have?
In mineralogy, ordinary table salt or rock table salt is called halite. The history of this transparent stone goes back millions of years. Halite forms in the form of cubic crystals, the color of which can vary from colorless to pinkish or yellow. The color of this mineral is associated with the type of impurities present in its thickness.
Halite can be found in the wild most often in layers of chemogenic sedimentary rocks, as well as in the bottom sediments of drying lakes and estuaries.
The main physical properties of salt are:
- ability to dissolve in water;
- the ability to crystallize on objects;
- salty taste;
- density - 2.165 g/cm3 at a temperature of 20 ° C;
- melting point - 801 °C;
- boiling point - 1413 ° C;
- solubility in water - 359 g/l at 20 °C.
NaCl has a pronounced taste. But no one will ever be able to smell the salt. This substance has a low hardness on the Mohs scale, and it is also brittle. Small particles of salt, for example, in places where it is found, can fly in the air and even get into a person’s nose. However, humans do not have receptors responsible for the perception of this substance. Some people claim that they can smell salt. However, in this case we are still not talking about NaCl, but about various kinds of impurities contained in this substance.
Table with comparison scale
Comparison of the strength characteristics obtained using various methods with the Mohs table is useful in the practical use of stones. Absolute values of hardness are determined by other estimates; a comparison of the criteria can be seen in the table.
| Minerals and rocks | Mohs grading | According to Schreiner, MPa | Strength on the Knoop scale, units | Grinding hardness in water according to Rozival | Strength according to microhardness tester PMT-2, PMT-3, kg/mm² |
| Talc | 1 | 250―400 | 12 | 0,03 | 2,4 |
| Gypsum | 2 | 32 | 1,25 | 40 | |
| Calcite, marble, anhydrite | 3 | 950―1400 | 135 | 4,5 | 110 |
| Fluorite, dolomite | 4 | 2500―3200 | 160 | 5 | 190 |
| Apatite, granite | 5 | 3000―3700 | 400 | 6,5 | 530 |
| Orthoclase, basalt | 6 | 3900 | 500 | 37 | 790 |
| Quartz, diabase | 7 | 6300 | 1250 | 120 | 1120 |
| Topaz | 8 | 1550 | 175 | 1430 | |
| Corundum | 9 | 1900 | 1000 | 2060 | |
| Diamond | 10 | 8300 | 140000 | 10060 |
The reason for the hardness of granite lies in its complex mineral composition, where the main components, quartz and mica, are classified into different categories - 7 and 2. Their quantitative ratio determines the properties of the rock.
The Mohs scale is also used to compare the hardness of metals - this is convenient when choosing a setting for a precious stone.
| Name | Tin | Gold Silver | Copper, bronze | Nickel | Platinum | Palladium | Titanium | Tungsten |
| Value by gradation | 1,5 | 2,5―3 | 3 | 4 | 4,5 | 4,7 | 6 | 7,5 |
The table shows which metals can scratch others. This circumstance is important to consider when storing jewelry together.
Solubility degree
The peculiarities of salt, among other things, include the fact that its solubility in water depends little on the temperature of the latter. This indicator for NaCl increases by 7 g from 0 to 100 °C. However, the solubility of the salt is significantly reduced if the water contains MgCl2 or CaCl2. This indicator increases sharply for NaCl with increasing pressure. The process of salt dissolution occurs with significant heat absorption. This substance is practically insoluble in alcohol.
Chemical properties
In terms of its composition, NaCl belongs to the group of medium salts. The chemical composition of table salt is as follows:
- Na - 39.34;
- Cl – 60.66.
In its pure form, the composition of this substance fully corresponds to the theoretical one. Table salt contains Br (up to 0.098%) as an isomorphic impurity. Halite may also contain: NH3, He, As, J, Pb and some other substances. The atoms in the Na and Cl structure alternate evenly at the sites of the cubic crystal lattice.
Salt crystals can reach significant sizes. Halite is also characterized by skeletal formations - fragile, cloudy-white pyramidal boats.
Methods of artificial production
Rock salt for the food industry or, for example, medicine can be obtained using different technologies. In laboratories, brines of underground dissolution of rock salt are usually used to isolate NaCl. This allows you to get the purest possible product without industrial impurities. In this case, underground brines are subjected to conventional evaporation. In this case, pure salt is obtained with a hardness on the Mohs hardness scale of 2. Evaporation of brines using this technique is carried out in special multi-vessel installations.
Hardness measurement methods
All methods for determining the hardness of metals use mechanical action on the test sample - indentation of an indenter. But this does not destroy the sample.
The Brinell hardness method was the first to be standardized in materials science. The principle of testing samples is described above. It is subject to GOST 9012. But you can calculate the value using the formula if you accurately measure the imprint on the sample:
HB=2P/(πD*√(D 2 -d 2 ),
- where P is the applied load, kgf;
- D – ball circumference, mm;
- d – imprint circumference, mm. The ball is selected relative to the thickness of the sample. The load is pre-calculated from accepted standards for the relevant materials: iron alloys - 30D 2; copper and its alloys - 10D 2; babbitts, lead bronzes - 2.5D 2.
Symbol of the test principle
Schematically, the Rockwell research method is depicted as follows according to GOST 9013.
Rockwell hardness test method
The final applied load is equal to the sum of the initial load and that required for the test. The device indicator shows the difference in penetration depth between the initial load and the test load h –h0.
The Vickers method is regulated by GOST 2999. It is schematically depicted as follows.
Mathematical formula for calculation: HV=0.189*P/d 2 MPa HV=1.854*P/d 2 kgf/mm 2 The applied load varies from 9.8 N (1 kgf) to 980 N (100 kgf). The values are determined from the tables relative to the measured print d.
The method is considered empirical and has a wide range of readings. But the device has a simple design and can be used when measuring large-sized and curved parts.
The Mohs hardness of metals and alloys can be measured by scratching. Mohs at one time proposed making scratches on the surface of an object with a harder mineral. He classified known minerals by hardness into 10 positions. The first is occupied by talc, and the last by diamond.
After measurement using one method, transfer to another system is very conditional. Clear values exist only in the Brinell and Rockwell hardness ratios, since machine-building enterprises widely use them. The dependence can be observed when the diameter of the ball changes.
| d, mm | HB | HRA | H.R.C. | HRB |
| 2,3 | 712 | 85,1 | 66,4 | — |
| 2,5 | 601 | 81,1 | 59,3 | — |
| 3,0 | 415 | 72,6 | 43,8 | — |
| 3,5 | 302 | 66,7 | 32,5 | — |
| 4,0 | 229 | 61,8 | 22 | 98,2 |
| 5,0 | 143 | — | — | 77,4 |
| 5,2 | 131 | — | — | 72,4 |
As can be seen from the table, increasing the diameter of the ball significantly reduces the readings of the device. Therefore, machine-building enterprises prefer to use measuring instruments with the same type of indenter size.
If you find an error, please select a piece of text and press Ctrl+Enter.
The hardness of a metal is its property of resisting plastic deformation during the contact action of a standard tip body on the surface layers of the material.
Read also: Propane RDSG reducer with adjustment
Hardness testing is the main method for assessing the quality of heat treatment of a product.
Determination of hardness using the Brinell method . The method is based on inserting a steel ball into a flat surface under load. Hardness number HB
determined by the ratio of the load to the spherical surface of the print.
The Rockwell (HR) method is based on statically pressing a tip into the test surface under a certain load. Steel balls are used as tips for materials with hardness up to 450 HR. In this case, the hardness is designated as HRB
.
When using a diamond cone, hardness is designated as HRA
or
HRC
(depending on load).
Vickers hardness (HV) is determined by statically pressing a diamond tetrahedral pyramid into the test surface. During the test, the indentation is measured with an accuracy of 0.001 mm using a microscope, which is an integral part of the Vickers instrument.
Shor's method . The essence of this method is to determine the hardness of the sample material by the height of the rebound of the striker falling onto the surface of the test body from a certain height. Hardness is assessed in conventional units proportional to the height of the striker's rebound.
Interesting Facts
The hardness of salt on the Mohs hardness scale is accurately determined. This indicator for NaCl is 2. People started thinking about the physical and chemical properties of salt not so long ago. But man has used this substance for various purposes since ancient times. First of all, salt has always been used, of course, primarily as a food product. However, sometimes she could perform other functions in society. For example, in Ethiopia this substance was used as currency until the 20th century.
In the Middle Ages, salt was so expensive that it was sometimes called white gold. In Germany, for example, there is still a special “salt pavement” along which this valuable food product was once transported between cities located on the shores of the Baltic Sea.
Salt is indeed a very important product for the human body. If you drink very large amounts of water, this substance will be washed out of the tissues. In this case, a person may even experience lethal hyponatremia.
Lack of salt in the human body is therefore very dangerous. But an excess of this substance, of course, cannot be useful. You should never eat too much salt at one time. Taking this substance in an amount of 1 g per 1 kg of weight can lead to death.
Source
Natural stones and the Mohs hardness scale
“Everything is known by comparison” - this phrase of Rene Descartes has long been perceived as folk wisdom. It is human nature to look for and find similarities and differences, and he does this constantly, with pleasure and benefit for himself. Comparison helps you make decisions and choices, gives you tools and teaches you how to use them.
The difference from touching ice, water and steam at the level of comparison of sensations is known to everyone. Anders Celsius based his temperature measurement scale on this, and since then humanity has been counting temperature from the freezing point of water.
Karl Friedrich Moos is a professor of mineralogy at the University of Graz (Austria). While studying the mechanical characteristics of minerals and rocks, he compared them in pairs, trying to damage the surface of one of them with the sharp edge of the other. The result of the research was the Mohs hardness scale - a simple and convenient tool for qualitative comparison of materials.
Principle of scale construction
This simple method, like all ingenious inventions, is based on the principle of pairwise comparison of a sample, the hardness of which is to be determined, with a standard series of minerals. The main difficulty was in selecting the original sequence of materials and arranging them in a strict order of increasing or decreasing hardness. To solve this problem, the best idea was to compare samples by attempting to scratch one of them on the surface of the other.
The experiments carried out helped the brilliant scientist not only construct a simple and logical scale, but also use minerals that are widespread in nature for it. Today, a standard set of talc, gypsum, calcite, fluorite, apatite, spar, quartz, topaz, corundum and diamond can be used by a university student in a teaching laboratory or a geologist on an expedition to the ends of the world.
Despite the very approximate accuracy of determining hardness, the Mohs scale is very popular and is used all over the world.
It should be remembered that the scale is not proportional and, for example, the hardness of corundum and its neighboring diamond in absolute units differs by 140 times, and that of other neighbors on the scale of calcite and fluorite by only 0.5 units.
Possible applications
The use of Professor Mohs' technique for practical applications involves the sequential use of minerals from a reference set to cause damage in the form of a scratch to the test material. So granite (depending on the quartz content in it) can scratch apatite (hardness 5) and feldspar (hardness 6), but it itself can be scratched by quartz, which allows it to be assigned a value of 5.5-6.5 on the scale, and garnit gabbrodiabase is equal in hardness to quartz – 7.0.
This simple measurement technique allows geologists to use the technique in the field to identify minerals.
Jewelers use it to choose the method of processing precious stones, which depends on their hardness, as well as to select the appropriate tool and grinding material. Another application is to identify counterfeit gemstones - they always have a lower hardness than the original stones.
In construction, knowledge of hardness on the Mohs scale helps to select an adequate replacement for a missing or currently unavailable material. the granite paving stones laid out in the project on the pavement can be replaced with gabbro paving stones , which have a higher hardness value.
In everyday practice, understanding the capabilities and characteristics of stones in jewelry and costume jewelry will allow you to properly organize their storage and use, and avoid scratches and other damage.
Relative and absolute hardness
In addition to the qualitative comparison of materials by their relative hardness in materials science, and mineralogy in particular, quantitative methods are also used to determine absolute hardness values. These methods, mainly borrowed from metallography, are based on measuring the dimensions of the depression that is formed in the test mineral (metal) when pressure is applied to its surface with a metal ball or diamond pyramid.
The first of them (using a ball) was proposed by the Swede Brinell and embodied in devices called Brinell presses. The second (with a diamond pyramid) was developed by the English concern Vickers Ltd. and received the name - the Vickers method. Absolute hardness is measured on these scales in kgf/mm2, and a device in which a steel or diamond needle scratches the test sample is called a sclerometer.
Another way to quantify hardness is the Rozival method. It is based on measuring the volume of a material sample as a result of grinding one of its faces. Grinding hardness is the reciprocal of the volume of material abraded. The hardness of quartz is taken as the standard, which corresponds to a value of 100 units on this scale.