Many people are interested in silver - a chemical element that is associated with luxury, expensive products, and unique technologies in science and technology. This element occupies a special place in the entire periodic table, since it has always, literally throughout the entire history known to mankind, been a material for the manufacture of coins, unique household items and other valuable things. Silver coins have been known since the most ancient civilizations, and they continue to be issued in our time.
The material also plays an important role in science, medicine, industry, and unique technologies, where serious requirements are placed on the purity of the substances used. Many people are interested not so much in the chemistry of silver, but in the various beliefs, legends and fairy tales associated with it. This also occupies a large layer of universal human culture and shows the importance of the element in question for people.
From Macedonski to Sapkowski
In the troops of Alexander the Great, military leaders were sick less often than privates. But both the food and the conditions during the hikes were the same for everyone. The reason for good health (as medical historians suggest) is in the dishes. The rank and file ate from tin plates, while the military leaders were given silver plates.
Our Russian ruble is inextricably linked with silver. No, there is currently no precious metal in ruble and other consumable coins. But the coin got its name from the word “chopping.” In Ancient Rus', silver ingots or rods were in circulation; they were called hryvnias. A piece was cut off from them (corresponding to the price of the product), and it was called a ruble.
Mystical powers have always been attributed to the precious metal. Protection from any evil spirits (even vampires and werewolves, who could only be killed by silver weapons), from the evil eye and other horrors of the night.
Those who have read Sapkowski's books about the witcher Herald (or played computer games based on the book) know: the witcher's sword was made of pure silver metal. After all, the Rivan’s profession was the destruction of all kinds of evil spirits.
How to determine a sample by density?
A method for determining the proportion of silver in an alloy was invented by Archimedes. The meaning of this non-destructive testing method is based on weighing the metal in water. In order to determine density, you need to know two quantities - volume and mass.
The weight of the product is determined by weighing on a scale. But how to determine the volume? To do this, we measure the volume of water displaced by the product. Divide mass by volume and get density. Using the table, we determine which metal sample the product belongs to.
Using the same method, you can distinguish other metals from each other. For example, the latest technology in the field of costume jewelry is the production of stainless steel products. To the untrained eye, it can be difficult to determine what kind of white metal a particular piece of jewelry is made of, but this can easily be determined by its density. Steel is much lighter than silver for the same volumes.
Chemistry and physics of noble metal
Silver has amazing properties.
It is the champion in electrical conductivity among metals. This metal has the highest thermal conductivity. The reflection of infrared and light waves by silver reaches 99%.
The density of lunar metal is 10.5 g/cm³. It is heavier than copper, but lighter than lead.
The hardness of silver is 2.5-3; it is a soft, malleable, ductile metal.
| Properties of the atom | |
| Name, symbol, number | Silver / Argentum (Ag), 47 |
| Atomic mass (molar mass) | 107.8682(2)[2] a. e.m. (g/mol) |
| Electronic configuration | [Kr] 4d10 5s1 |
| Atomic radius | 144 pm |
| Chemical properties | |
| Covalent radius | 134 pm |
| Ion radius | (+2e) 89 (+1e) 126 pm |
| Electronegativity | 1.93 (Pauling scale) |
| Electrode potential | +0,799 |
| Oxidation states | 2, 1 |
| Ionization energy | 1st: 730.5 kJ/mol (eV) 2nd: 2070 kJ/mol (eV) 3rd: 3361 kJ/mol (eV) |
| Thermodynamic properties of a simple substance | |
| Density (at normal conditions) | 10.5 g/cm³ |
| Melting temperature | 1235.1 K; 962 °C |
| Boiling temperature | 2485 K; 2162°C |
| Ud. heat of fusion | 11.95 kJ/mol |
| Ud. heat of vaporization | 254.1 kJ/mol |
| Molar heat capacity | 25.36[3] J/(K mol) |
| Molar volume | 10.3 cm³/mol |
| Crystal lattice of a simple substance | |
| Lattice structure | cubic face-centered |
| Lattice parameters | 4.086 Å |
| Debye temperature | 225 K |
| Other characteristics | |
| Thermal conductivity | (300 K) 429 W/(mK) |
| CAS number | 7440-22-4 |
General information:
| 100 | General information | |
| 101 | Name | Silver |
| 102 | Former name | |
| 103 | Latin name | Argentum |
| 104 | English name | Silver |
| 105 | Symbol | Ag |
| 106 | Atomic number (number in table) | 47 |
| 107 | Type | Metal |
| 108 | Group | Precious, transition metal, platinum group metal |
| 109 | Open | Known since ancient times |
| 110 | Opening year | before 5000 BC |
| 111 | Appearance, etc. | Malleable, ductile silver-white metal |
| 112 | Origin | Natural material |
| 113 | Modifications | |
| 114 | Allotropic modifications | |
| 115 | Temperature and other conditions for the transition of allotropic modifications into each other | |
| 116 | Bose-Einstein condensate | |
| 117 | 2D materials | |
| 118 | Content in the atmosphere and air (by mass) | 0 % |
| 119 | Content in the earth's crust (by mass) | 7,9·10-6 % |
| 120 | Content in seas and oceans (by mass) | 1,0·10-8 % |
| 121 | Content in the Universe and space (by mass) | 6,0·10-8 % |
| 122 | Abundance in the Sun (by mass) | 1,0·10-7 % |
| 123 | Content in meteorites (by mass) | 0,000014 % |
| 124 | Content in the human body (by weight) |
Loot: we take it where we find it
Gold and silver are found in the form of nuggets. Therefore, both metals have been used by people since ancient times, and silver was often valued more than gold.
We recommend: CADMIUM - toxic, heavy and rare
In the 15th century, on the border between Saxony and Bohemia, a block of pure native silver weighing 20 tons was discovered.
The Canadian nugget was almost 30 meters long and weighed about 20 tons. Because of its shape, it was called the “silver sidewalk.”
But such cases are rare; Silver in its native state is less common than gold.
Most often, silver ores occur in the form of veins and mineralized zones in volcanic belts.
Lunar metal can be mined in:
- Peru;
- Mexico;
- Chile;
- Germany;
- USA;
- China;
- Russia.
- Australia.
Many countries do not advertise statistics on the production of the precious metal. But what is certain is that the demand for it is increasing, and we should expect an increase in its production in the world.
Only a quarter of silver is mined directly from silver deposits. The rest of the metal is obtained as a by-product from the mining of gold, copper, and lead.
More than 50 natural silver minerals are known, of which only 15–20 are of industrial importance:
- native silver;
- electrum (gold-silver);
- kustelite (silver-gold);
- argentite (silver-sulfur);
- proustite (silver-arsenic-sulfur);
- bromargerite (silver-bromine);
- kerargyrite (silver-chlorine);
- pyrargyrite (silver-antimony-sulfur);
- stephanite (silver-antimony-sulfur);
- polybasite (silver-copper-antimony-sulfur);
- freibergite (copper-sulfur-silver);
- argentoyarosite (silver-iron-sulfur);
- dyscrazite (silver-antimony);
- aguilarite (silver-selenium-sulfur).
Largest silver deposits in the world
The distribution of the precious resource across the surface of the planet is extremely uneven. The most intensive extraction of material occurs in Peru
– this state is the undisputed leader in the amount of silver produced here. On average, about 110 million ounces of the substance are mined in this country in one year.
Poland is also one of the leaders in silver mining, although few people realize this. About 40.5 million ounces of silver are mined here.
Russia and post-Soviet countries have about 12-15% of the world's silver reserves. The mining industry here is quite developed and produces tons of material annually. Australia is also included in the list of lucky ones who are lucky with the resource in question. There is not much silver in other parts of the world, but there is always the possibility of discovering as yet unknown deposits.
Application
Noble metal works for the needs of people. In the production of electrical and electronic products, in solders for critical products, in the jewelry industry.
Silver iodide is used to disperse clouds (and you wonder why the weather is always good in the capital during the holidays...).
Used to disinfect water, but on a large scale it is not economically viable.
For high quality mirrors, silver is used (simple mirrors use aluminum). Mirrors with precious metal are used to supply solar energy to desalination plants in Saudi Arabia (they have a lot of money, but fresh water is tight).
Food additive E174 is silver; used in the preparation of desserts and culinary products (expensive, it must be said, products). If you are a fan of “silver food delights,” do not forget: the permissible daily dose of metal is no more than 7 milligrams per day.
This is a small list of places where you cannot do without precious metal.
Photographic equipment and electronics
The use of silver nitrate and halide for making photographs has recently lost its relevance, because with the advent of digital technology, the demand for color film began to fall rapidly. Global demand for photographic silver peaked in 1999. Now, outdated film photographic equipment is gradually disappearing even in third world countries.
In electrical products, this metal is used for its excellent conductivity. Some electrical manufacturers produce connecting, power and speaker cables using the highest quality silver conductors.
Their conductivity is 6% higher than that of identical copper analogues, but they are also noticeably more expensive. Oddly enough, the demand for such products is quite high. Many audiophile enthusiasts claim that such conductors in audio systems improve sound quality, but this has not been scientifically proven.
Small devices such as watches and hearing aids typically use silver batteries. The reason is that the mass of this metal is relatively small, and its energy-saving properties are very good. It is also used to make silver-zinc and silver-cadmium batteries. In addition, cadmium silver oxide is used in high-voltage contacts.
We heal with lunar metal
People have known about the anti-inflammatory properties of silver for a long time. In America, Indians disinfected water with pieces of hot metal. And the white settlers of the New World put a silver coin in a pot of milk to prevent it from turning sour.
Silver coins
Interesting: in Russia, for this purpose, they took a frog from the nearest pond. And for good reason - research by chemists from Moscow State University has proven that amphibians secrete antimicrobial and antifungal substances into their skin.
Modern silver preparations (collargol, protargol) relieve:
- erysipelas;
- purulent conjunctivitis;
- rhinitis;
- purulent wounds.
The healing properties of the noble metal include the antiseptic and healing properties.
Caution: prolonged use of drugs with silver threatens argyrosis. With this disease, the skin and mucous membranes take on a silvery or bluish-gray color. Colloidal silver taken long-term and in large doses can cause pulmonary edema, bone marrow dysfunction, and even coma. We recommend: COBALT - a generous gift from mountain spirits
Silver, like its patroness the Moon, favors women. Therefore, jewelry made from lunar metal will be useful for women during PMS who are prone to hysteria and inappropriate behavior.
Water has long been infused with silver and drunk. This water is useful for gastrointestinal diseases and throat diseases. Problems with the cardiovascular system should be treated by a doctor, but wearing silver jewelry (as esotericists say) will be useful.
Silver is now again attracting the attention of doctors. Water infused with a noble metal, if taken constantly, strengthens the immune system and does not suppress the intestinal microflora (which is the sin of all antibiotics).
And the ability of the precious metal to heal wounds and cuts has been used by doctors for a long time.
Silver water has a beneficial effect on the skin. It’s not for nothing that cosmetic companies produce product lines with “white gold”.
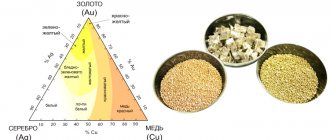
Chemical properties of silver
Silver is a relatively soft and ductile metal; from 1 g of it you can draw a metal thread 2 km long! Silver is a heavy metal and has low thermal and electrical conductivity. The melting point is relatively low, only 962° C. Silver readily forms alloys with other metals, which give it new properties, for example, by adding copper, a harder alloy is obtained - billon. Under normal conditions, silver is not subject to oxidation, but has the ability to absorb oxygen. When heated, solid silver can dissolve five times the volume of oxygen! An even larger volume of gas is dissolved in liquid silver, approximately 20:1. Iodine can attack silver. The noble metal is especially “afraid” of iodine tincture and hydrogen sulfide. This is the reason why silver darkens over time. Sources of hydrogen sulfide in everyday life include spoiled eggs, rubber, and some polymers. When hydrogen sulfide and silver react, especially at high humidity, a very strong sulfide film is formed on the metal surface, which is not destroyed by heating and exposure to acids and alkalis. It can only be removed mechanically, for example with a brush with toothpaste applied to it. The biochemical properties of silver are interesting. Despite the fact that silver is not a bioelement, it can influence the vital activity of microbes by suppressing the work of their enzymes. This occurs when silver combines with an amino acid that is part of the enzyme. Therefore, water in silver vessels does not spoil, because it inhibits the activity of bacteria.
Silver magic
The magical properties of the noble metal are recognized even by the church. Epiphany water is blessed with a silver cross; this water is healing. It is good for every baptized person to wear a silver cross.
The magic of silver absorbs negativity directed at the owner of the jewelry. If a precious metal cannot cope with the flow of negative energy (think about who you have annoyed so much), it darkens or even turns black. By the way, this also happens if the owner of the silver jewelry is sick. In any case, it is advisable to visit a doctor and esotericist.
If possible, place a silver figurine, candlestick, or vase in your apartment. This will clear the room of negativity. Don’t forget to inspect the product - it has darkened, changed color - it has accumulated negativity, it’s time to do a “magic cleaning”.
Silver vases
The custom of giving a baby a silver spoon as a gift is useful, researchers of the precious metal have no doubt about it.
Silver spoons
For women, wearing lunar metal bracelets means enhancing their feminine attractiveness. Earrings will help cope with migraines and headaches.
A belt with a silver buckle will help men and women improve their health. Just make sure the buckle is just below your belly button.
Melting alloys
When melted, it easily reacts with oxygen, so it is melted in a covered graphite crucible. The crucible is preheated to remove dirt and moisture. If a ceramic crucible is used, then coal is added to it during melting. Phosphorus or phosphorous copper may be added to prevent oxidation of the alloy.
If a graphite crucible is used, this is not necessary, since oxidation is minor and subsequent cleaning will remove it. The appearance of the surface of the silver when cast can provide vital information - at the right temperature it has a pinkish tint, the surface is clean and shiny. After melting, the top layer, which contains copper oxides, is removed. It is important not to overheat the alloy as this may affect its stability and uniformity.
Star preferences for lunar metal
Astrologers have always associated silver with our satellite the Moon. The purity, whiteness, secrecy and discreetness of the precious metal cannot but be associated with the same qualities of the Moon.
For zodiac signs, lunar metal is acceptable and useful. However, it will have the greatest influence on the signs of Water (these are Pisces, Scorpios, Cancers - their night ruler, the Moon). The “Wolf Sun,” as some peoples call the Moon, will provide water signs with subtle intuition and the ability to get around “sharp corners.”
Air signs will also receive the support of the night luminary. Just remember to take a break from lunar decorations sometimes.
We recommend: ALUMINUM - the roads it chooses
Earth and Fire signs can be treated with silver. But they are not recommended to constantly wear jewelry made of precious metal.
Examples of problem solving
| Exercise | When 24.6 g of the substance was burned, 26.88 liters of carbon dioxide (at standard conditions), 9 g of water and 2.24 liters of nitrogen (at standard conditions) were formed. 1 liter of vapor of this substance (at standard conditions) has a mass of 5.491 g. Based on the data of the task conditions, carry out the necessary calculations and establish the molecular formula of the original organic substance. |
| Solution | Let’s draw up a diagram of the combustion reaction of an organic compound, designating the number of carbon, hydrogen and oxygen atoms as “x”, “y” and “z”, respectively: |
Let us determine the masses of the elements that make up this substance. Values of relative atomic masses taken from the Periodic Table of D.I. Mendeleev, round to whole numbers: Ar(C) = 12 amu, Ar(H) = 1 amu, Ar(O) = 16 amu, Ar(N) = 14 amu
Let's calculate the molar masses of carbon dioxide and water. As is known, the molar mass of a molecule is equal to the sum of the relative atomic masses of the atoms that make up the molecule (M = Mr):
M(CO2) = Ar(C) + 2×Ar(O) = 12+ 2×16 = 12 + 32 = 44 g/mol;
M(H2O) = 2×Ar(H) + Ar(O) = 2×1+ 16 = 2 + 16 = 18 g/mol.
Let's determine the chemical formula of the compound:
x:y:z:w = m(C)/Ar(C) : m(H)/Ar(H) : m(N)/Ar(N) : m(O)/Ar(O)
x:y:z:w= 14.4/12 : 1/1 : 1.28/14 : 6.4/16;
x:y:z:w= 1.2: 1: 0.09: 0.4 = 13: 11: 1: 4.
This means the simplest formula of the compound is C13H11NO4 and the molar mass is 30 g/mol [M(C13H11NO4) = 13×Ar(C) + 11×Ar(H) + Ar(N) + 3× Ar(O) = 13×12 + 11×1 + 14 + 3×16 =156 + 11 + 14 + 48 = 229 g/mol].
Let's find the true molar mass of an organic substance:
To find the true formula of an organic compound, we find the ratio of the true and resulting molar masses:
This means that the indices of carbon, hydrogen and oxygen atoms must be multiplied by 0.5, i.e. the formula of the substance will be C6H5NO2. This is nitrobenzene.
| Exercise | A monocarboxylic acid contains 26.1% carbon and 4.3% hydrogen. Based on the data from the task conditions, make the necessary calculations and establish the molecular formula of the organic substance. |
| Solution | Let us write the molecular formula of a monobasic carboxylic acid in general form as CxHyOw. |
Then, the mass fraction of oxygen in the composition of a saturated monobasic carboxylic acid will be calculated as:
We divide the percentage content of elements into the corresponding relative atomic masses (the values of relative atomic masses taken from D.I. Mendeleev’s Periodic Table are rounded to whole numbers). Thus we will find the relationship between the number of atoms in the molecule of the compound:
x:y:w = ω(C)/Ar(C) : ω (H)/Ar(H) : ω(O)/Ar(O);
x:y:w: = 2.18: 4.3: 4.35 = 1: 2: 2.
This means that the simplest formula of a monobasic carboxylic acid will be CH2O2 or HCOOH. This is formic acid.
Source
Let it be as bright as the moon
For silver, unfavorable factors are:
- human sweat;
- cosmetic and medicinal preparations containing sulfur, iodine;
- taking vitamin and mineral supplements;
- high temperature (bath, sauna).
All this can harm the color and appearance of the silver item.
Cleaning silver at home is easy. Hold the product in soapy water, rinse, and wipe dry with flannel or suede.
Take your jewelry to a jeweler at least once a year. It will restore the shine and pristine beauty of your favorite trinket using special means.
Good to know: do not try on other people's silver jewelry and do not let them measure your own.
The effect of silver on humans
As we saw above, the use of small doses of silver has a disinfecting and bactericidal effect. However, what is beneficial in small doses is very often harmful in large doses. Silver is no exception here. An increase in the concentration of silver in the body can cause a decrease in immunity, damage to the kidneys and liver, thyroid gland and brain. In medicine, cases of mental disorders due to silver poisoning have been described. Long-term intake of silver into the body in small doses leads to the development of argyria. The metal is gradually deposited in the tissues of organs and gives them a greenish or bluish color, this effect is especially visible on the skin. In severe cases of argyria, the skin darkens so much that it resembles the skin of Africans. Apart from the cosmetic effect, argyria does not otherwise cause any deterioration in well-being or disruption of the body’s functioning. But there is also a plus here, given that the body is saturated with silver, it does not care about any infectious diseases!
American Paul Carson "Papa Smurf", who suffered from argyria
Jewelry delights
The use of lunar metal in jewelry fashion is understandable and justified. The discreet beauty of the metal allows you to wear silver jewelry with almost any clothing; reasonable prices make such jewelry accessible to many.
How to decorate silver
There are different coatings to highlight the jeweler's idea. This:
- Rhodium plating is the coating of a product with the thinnest layer of rhodium. As a result, the decoration always remains light and shiny. Many connoisseurs of silver products believe that this shine is unnatural. For those who wear silver, such products are useless for protection against damage.
- Blackening. Some jewelry requires an "antique look." So a layer of “niello” (a mixture of copper, sulfur, and silver sulfides) is applied to the product.
- Oxidation. Preventive and controlled oxidation of jewelry. A layer of silver oxide is applied to its surface, the product darkens slightly and evenly, but the metal does not oxidize during wear.
- Gilding. The oldest way to decorate products made from our precious metal. A thin layer of gold is applied to the surface. However, gold-plated products require careful care and operation.
Origin of the name
Silver is a metal that has attracted the attention of people since ancient times. The name "silver" comes from the Sanskrit word "argenta". It means "light". The Latin "argentum" (silver) has the same roots. But in this language it means "white."
Silver is a noble metal, and alchemists did not ignore it. In ancient times, they developed a method for eliminating this natural element.
In Russian, the metal in question is called “silver”, in English it sounds like “silver”, in German – “silber”. All these words come from the ancient Indian “sarpa”, meaning Moon. The explanation for this is quite simple. The shine of silver reminded people of the light of a mysterious celestial body.