Argentum, or silver, is a metal and chemical element assigned atomic number 47 in the periodic table. The chemical formula of the metal is Ag. Silver was explored by mankind back in the fourth millennium BC. The discovery of this metal did not require the help of scientists, since it was found by man as native silver. Moreover, the nuggets reached very impressive sizes. For example, in the fifteenth century a nugget weighing over 20 tons was mined.
However, mining silver required more effort than mining gold. For this reason, for several centuries, silver was more expensive than gold. The reserves of silver ore on Earth today amount to over 550 tons, and the leading countries in silver mining are considered to be:
- Peru.
- Australia.
- Chile.
- Mexico.
The precious metal is found in the Earth's crust in a concentration of 70 milligrams per ton. In nature, argentum is found in most cases in ore deposits in combination with other elements. There are over fifty types of silver ores in nature, but the following are considered effective from an economic point of view:
- native silver;
- kustelite;
- electrum;
- bromargerite;
- will be sad;
- aguilarite and others.
Silver can occur in nature together with gold, and this formation is called electrum. The noble metal is concentrated in large quantities in ores containing uranium, bismuth and nickel.
Silver crystals
Native silver is found in sulfide ores, in which it forms tiny crystals dispersed among the other metals of which the ores are composed. At the fractures, the crystals have an uneven angular surface, which makes them look like hooks. This is a find that is found in natural conditions much less often than gold. Moreover, the appearance of such nuggets is very unusual. Due to its plasticity, silver forms nuggets that resemble lattices, tubes, branches and strands. For this reason, such silver is not used for industrial purposes, but serves only as an exhibit in museums.
Silver, properties of the atom, chemical and physical properties.
Ag 47 Silver
107.8682(2) 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s1
Silver is an element of the periodic system of chemical elements of D.I. Mendeleev with atomic number 47. It is located in the 11th group (according to the old classification - a secondary subgroup of the first group), the fifth period of the periodic system.
Silver atom and molecule. Silver formula. Structure of the silver atom
Silver price
Isotopes and modifications of silver
Properties of silver (table): temperature, density, pressure, etc.
Physical properties of silver
Chemical properties of silver. Interaction of silver. Chemical reactions with silver
Getting silver
Application of silver
Table of chemical elements D.I. Mendeleev
Being in nature
If we consider the composition of the earth's crust, then it contains approximately 70 milligrams of silver per 1 ton. It's not that much. Since ancient times, silver coins and objects have had a high value, which already indicates that the material is rare and valuable.
Alloys of the material are less common than pure ore, but with the current development of technology they are easily processed to isolate the required fraction.
It is interesting to note that often the diggers were able to discover not ore, but nuggets, and of enormous size. History knows a case when a silver nugget weighing as much as 20 tons was found!
Other native objects weighing 500-600 kilograms were also found.
Silver atom and molecule. Silver formula. Structure of the silver atom:
Silver (lat. Argentum) is a chemical element of the periodic system of chemical elements of D.I. Mendeleev with the designation Ag and atomic number 47. It is located in the 11th group (according to the old classification - a secondary subgroup of the first group), the fifth period of the periodic system.
Silver is a metal. Belongs to the group of transition metals, as well as precious metals and platinum group metals.
Silver is represented by the symbol Ag.
As a simple substance , silver under normal conditions is a malleable, ductile metal of a silvery-white color.
The silver molecule is monatomic.
The chemical formula of silver is Ag.
The electronic configuration of the silver atom is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s1. The ionization potential (first electron) of the silver atom is 731 kJ/mol (7.576234(25) eV).
Structure of the silver atom. The silver atom consists of a positively charged nucleus (+47), around which 47 electrons move through five shells. In this case, 46 electrons are in the internal level, and 1 electron is in the external level. Since silver is located in the fifth period, there are only five shells. First, the inner shell is represented by the s-orbital. The second - the inner shell is represented by s- and p-orbitals. The third and fourth - inner shells are represented by s-, p- and d-orbitals. The fifth - outer shell is represented by the s-orbital. At the outer energy level of the silver atom, there is one unpaired electron in the s orbital. In turn, the nucleus of a silver atom consists of 47 protons and 61 neutrons. Silver belongs to the elements of the d-family.
The radius of the silver atom (calculated) is 165 pm.
The atomic mass silver atom is 107.8682(2) a. eat.
Silver , being a noble metal, is characterized by relatively low reactivity.
Sulfur, atomic properties, chemical and physical properties
History of metal
A substance such as silver has been known to mankind for many millennia, if not hundreds of thousands of years. In the most ancient myths, legends and stories you can find references to money and objects made from it. The material received its name from the Proto-Slavic dialect, which was widespread in the territory of present-day Russia, Germany and the Baltic countries.
Literally translated, it means “brilliant”, “white to shine.”
The reason for the substance's early prominence in many cultures is that, unlike other metals that are mined by smelting, the ore of the material in question does not require this procedure. Very often, ancient people encountered silver in the form of nuggets ready for processing. That is, no complex technologies were needed to simply take it and actively use it for one’s own purposes.
Superstitions
The mystical connection with the moon, aesthetic qualities and the fact that silver symbolizes purity are the reasons why it has traditionally been considered an antidote to various ailments and imaginary monsters in European folklore. It is noteworthy that they used it to fight vampires and the living dead. It was believed that only a weapon or a silver bullet could kill a werewolf in his bestial form. According to Eastern European folklore, silver bullets were an effective weapon against vampires. These beliefs gave rise to the term, which is used to describe drugs intended to treat or eliminate a wide range of diseases.
Top countries by production
The most recent information I was able to find is for 2017. The list of leaders in silver mining is as follows:
- Mexico - 6108 tons;
- Peru – 4587 tons;
- China - 3502 tons;
- Russia – 1306 tons;
- Chile – 1260 tons;
- Bolivia - 1244 tons;
- Poland - 1228 tons;
- Australia - 1101 tons;
- USA - 1048 tons;
- Argentina – 823 tons.
It is known that the volume of production of this metal around the world continues to decline for the third year in a row. We look forward to the latest results.
Interesting Facts
Europeans found vast amounts of silver in the New World, such as Zacatecas in Mexico and Potosí in Bolivia. They say that the conquistador Francisco Pizarro shoed his horses with it, there was so much of this metal. In contrast, Peru experienced a relative iron deficiency. However, silver was an extremely valuable metal in other places. This made it a global commodity, causing inflation in Europe and contributing to the rise of the Spanish Empire. The rise and fall in the value of silver has affected the global market.
The Rio de la Plata River is named after silver. In addition, Argentina gets its name from the Latin name for this valuable metal.
The search for silver and related metals (particularly lead) in the galenic ore in which it is most commonly found contributed to the settlement of western North America. The famous "silver rush" occurred in Colorado, Nevada, California, Ontario and the Kootenay Regional District of British Columbia. The largest metal deposits in the United States were discovered in Virginia (Nevada) in 1859.
Areas of application
About 80% of the mined metal is consumed by the industrial sector of the economy. The remaining 20% goes to jewelry production, medical needs and investments.
It is difficult to list all the industries where this metal is used, since the scope of its application is too broad. These are electronic equipment, optical instruments, material for medical equipment, weather control, pharmacology, dentistry and much more.
In Japan, silver is used in air conditioning devices; in America, pieces of metal are placed in milk to prevent it from souring.
In recent years, a significant increase in demand for silver has been demonstrated by the energy sector of the economy. This metal is used in the production of modern high-tech solar panels.
How to spot a fake
There are a couple of simple ways to quickly check the authenticity of a precious metal.
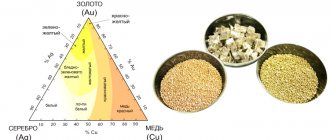
- Magnet - silver and copper are diamagnetic metals, so they should not be magnetized under any circumstances.
- Lapis pencil - will not leave marks on the metal, since it is silver nitrate, or, as it is designated in chemistry, AgNO3.
- Vinegar – Place the item in regular table vinegar. The real thing will not react in any way, and the fake will turn white.
- Heat - silver will instantly heat up, just put it in boiling water for a moment.
Tips for choosing silver jewelry
When choosing an item, base it on how you want to use it. It is better to choose tableware from metal with a lower grade, but durable. Everyday jewelry is made from sterling alloy, which is what they are most often made from. Items for special occasions can be chosen from high-quality metal: they will be used infrequently, but they will not require special care.
Precautionary measures
Silver has no natural biological function in humans, and the possible health effects of its use are a matter of debate. Ag metal itself is not toxic, but most of its salts are dangerous and some are carcinogenic.
Silver (especially colloidal) and its compounds can enter the circulatory system and be deposited in various tissues of the body, resulting in a condition called argyria. It causes pigmentation of the skin, eyes and mucous membranes. Although this condition is not harmful to a person's health, it usually does not go away. Argyria is rare, and its mild forms are sometimes mistaken for cyanosis.
Silver ions and compounds have a toxic effect on some bacteria, viruses, algae and fungi, similar to heavy metals such as lead or mercury. However, they are not as dangerous to humans as lead and mercury.
Reviews
Lydia:
About ten years ago I bought a silver bar, I recently sold it very profitably, I recommend silver as a financial investment.
Svetlana:
I have always loved silver jewelry more than gold, and they say it’s good for your health.
Gregory:
I bought silverware for the house, we use it, we continue the traditions of our ancestors, although my wife complains that she is tired of cleaning it.
The richest deposits
Silver deposits are almost evenly distributed across all continents, but undisputed leaders in its production have emerged in the world. I have compiled lists of the richest and most famous deposits to date.
In the world
The most promising deposits with annual silver production are as follows:
- Cannington (Australia), 1430 tons;
- Fresnillo (Mexico), 982 tons;
- Dukat (Russia), 375 t;
- Uchuchacua (Peru), 305 tons;
- Greens Creek (USA), 302 tons;
- Arcata (Peru), 247 tons;
- Rochester (USA), 176 tons;
- Imiter (Morocco), 153 tons;
- Juaron (Peru), 126 tons;
- Lunnoe (Russia), 115 t.
In Russia
Russia is one of the world leaders in silver mining today. The richest deposits with the best characteristics in terms of the amount of precious metal per ton of ore:
- Dukat (Magadan), 640 g/t;
- Lunnoe (Magadan), 404 g/t;
- Khakanjinskoye (Khabarovsk), 344 g/t;
- Goltsovoye (Magadan), 1213 g/t;
- Forecast (Yakutia), 876 g/t;
- Podolskoe (Bashkortostan), 28 g/t;
- Gorevskoye (Krasnoyarsk), 56 g/t;
- Ozernoye (Buryatia), 35 g/t;
- Uzelginskoye (Chelyabinsk), 30 g/t;
- Gayskoye (Orenburg), 11 g/t.
Recommendations for care, cleaning and storage
It is recommended to keep silver away from sunlight, in separate bags or cases. Clean the metal at the first sign of plaque, since over time it will become more and more difficult to restore the shine.
Such items require mechanical cleaning, since silver sulfide - that very dark coating on the product - does not dissolve much. To care for your jewelry, you can use toothpaste and a soft brush or napkin and carefully, without pressure, carry out the appropriate manipulations.