| Platinum | |
| Atomic number | 78 |
| Appearance of a simple substance | Heavy soft silver-white metal |
| Properties of the atom | |
| Atomic mass (molar mass) | 195.08 a. e.m. (/mol) |
| Atomic radius | 139 |
| Ionization energy (first electron) | 868.1(9.00) kJ/mol () |
| Electronic configuration | [Xe] 4f14 5d9 6s1 |
| Chemical properties | |
| Covalent radius | 130 |
| Ion radius | (+4e) 65 (+2e) 80 |
| Electronegativity (Pauling) | 2,28 |
| Electrode potential | Pt←Pt2+ 1.20V |
| Oxidation states | 4, 2, 0 |
| Thermodynamic properties of a simple substance | |
| Density | 21,45 /³ |
| Molar heat capacity | 25.85[1]/(mol) |
| Thermal conductivity | 71,6 /(·) |
| Melting temperature | 2045 |
| Heat of Melting | 21.76 kJ/mol |
| Boiling temperature | 4100 |
| Heat of vaporization | ~470 kJ/mol |
| Molar volume | 9.10 ³/mol |
| Crystal lattice of a simple substance | |
| Lattice structure | cubic face-centered |
| Lattice parameters | 3,920 |
| c/a ratio | n/a |
| Debye temperature | 230,00 |
| Pt | 78 |
| 195,08 | |
| 4f145d96s1 | |
| Platinum | |
Platinum - element 78 of the periodic table, atomic mass 195.08; noble metal of steel-gray color. Platinum was not known in the Old World, but the Andean civilizations (Inca and Chibcha) mined and used it since time immemorial. In Europe, platinum was unknown until the 18th century.
In 1735, the Spanish king issued a decree ordering that platinum should no longer be imported into Spain. When developing placers in Colombia, it was ordered to carefully separate it from the gold and drown it under the supervision of royal officials in the deep places of the Rio del Pinto River, which became known as the Platino del Pinto. And the platinum that had already been brought to Spain was ordered to be publicly and solemnly drowned in the sea. In 1748, the Spanish mathematician and navigator A. de Ulloa was the first to bring to the European continent samples of native platinum found in Peru. Platinum was first obtained in its pure form from ores by the English chemist W. Wollaston in 1803; the Italian chemist Gilius Scaliger discovered the indecomposability of platinum in 1835 and thus proved that it is an independent chemical element. In Russia, back in 1819, a “new Siberian metal” was discovered in alluvial gold mined in the Urals. At first it was called white gold; platinum was found at the Verkh-Isetsky, and then at the Nevyansky and Bilimbaevsky mines. Rich placers of platinum were discovered in the second half of 1824, and the following year its mining began in Russia.
Chemical composition of platinum
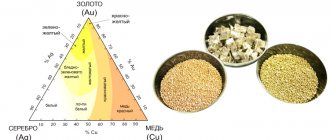
Pure platinum is very rare. Native platinum as a type is a solid solution containing more than 50% Pt (80–88%) with a metal of the same or other groups, mainly iron. Hence the original name of ordinary platinum, polyxenes (Greek for “receiving many guests”). The fusion (formation of solid solutions) of platinum metals is due to the proximity of their atomic radii, caused by “lanthanide compression”.
Most samples are represented by the ferrous variety (polyxene), and often by intermetallic compounds: isoferroplatinum (Pt,Fe)3Fe and tetraferroplatinum (Pt,Fe)Fe. Platinum, represented by polyxene, is the most common mineral of the platinum subgroup in the earth's crust. Fe is constantly present - up to 9.2% (in isoferroplatinum), sometimes reduced to 4–5% (the Pt content also changes accordingly). Of other isomorphic impurities, the following are established: Pd - 0.1–2.0%, sometimes up to 21% - palladium platinum; Ir - up to 7% - platinum iridide; Rh - 0.1–0.5%, sometimes up to 4–5% - native platinum; Cu - up to 0.8%; Ni - traces up to tenths of a percent, sometimes in very significant quantities - nickel platinum.
Varieties:
- ferroplatinum containing 10-30% Fe;
- cuproplatinum with 14% Cu;
- norilskite with 25% Fe and 26% Ni;
- nickel-platinum containing about 3% Ni;
- palladium platinum with 7-40% Pd;
- platinum iridium containing up to 70% 1g;
- native platinum with 5% Rh.
Valency of chemical elements (Table)
The valence of chemical elements is the ability of chemical atoms. elements form a certain number of chemical bonds. It takes values from 1 to 8 and cannot be equal to 0. It is determined by the number of electrons of an atom spent on the formation of a chemical. bonds with another atom. Valence is a real value. Indicated by Roman numerals (I ,II, III, IV, V, VI, VII, VIII).
How can you determine valence in compounds:
— The valency of hydrogen (H) is always constant 1. Hence, in the compound H2O, the valency of O is 2.
— The valency of oxygen (O) is always constant 2. Hence, in the CO2 compound, the valency C is equal to 4.
— The highest valency is always equal to the group number.
- The lowest valency is equal to the difference between the number 8 (the number of groups in the Periodic Table) and the number of the group in which the element is located.
— For metals in subgroups A of the periodic table, valency = group number.
— Nonmetals usually have two valences: higher and lower.
The valence of chemical elements can be constant or variable. Constant mainly for metals of the main subgroups, variable for non-metals and metals of secondary subgroups.
Table of valency of chemical elements
| Atomic no. | Chemical element | Symbol | Valency of chemical elements | Connection examples |
| 1 | Hydrogen | H | I | HF |
| 2 | Helium | He | absent | — |
| 3 | Lithium | Li | I | Li2O |
| 4 | Beryllium | Be | II | BeH2 |
| 5 | Bor / Boron | B | III | BCl3 |
| 6 | Carbon | C | IV, II | CO2, CH4 |
| 7 | Nitrogen / Nitrogen | N | I, II, III, IV | NH3 |
| 8 | Oxygen | O | II | H2O, BaO |
| 9 | Fluorine | F | I | HF |
| 10 | Neon / Neon | Ne | absent | — |
| 11 | Sodium/Sodium | Na | I | Na2O |
| 12 | Magnesium / Magnesium | Mg | II | MgCl2 |
| 13 | Aluminum | Al | III | Al2O3 |
| 14 | Silicon | Si | IV | SiO2, SiCl4 |
| 15 | Phosphorus / Phosphorus | P | III, V | PH3, P2O5 |
| 16 | Sulfur/Sulfur | S | VI, IV, II | H2S, SO3 |
| 17 | Chlorine | Cl | I, III, V, VII | HCl, ClF3 |
| 18 | Argon / Argon | Ar | absent | — |
| 19 | Potassium/Potassium | K | I | KBr |
| 20 | Calcium | Ca | II | CaH2 |
| 21 | Scandium / Scandium | Sc | III | Sc2S3 |
| 22 | Titanium | Ti | II, III, IV | Ti2O3, TiH4 |
| 23 | Vanadium | V | II, III, IV, V | VF5, V2O3 |
| 24 | Chrome / Chromium | Cr | II, III, VI | CrCl2, CrO3 |
| 25 | Manganese / Manganese | Mn | II, III, IV, VI, VII | Mn2O7, Mn2(SO4)3 |
| 26 | Iron | Fe | II, III | FeSO4, FeBr3 |
| 27 | Cobalt | Co | II, III | CoI2, Co2S3 |
| 28 | Nickel | Ni | II, III, IV | NiS, Ni(CO)4 |
| 29 | Copper | Cu | I, II | CuS, Cu2O |
| 30 | Zinc | Zn | II | ZnCl2 |
| 31 | Gallium | Ga | III | Ga(OH)3 |
| 32 | Germanium / Germanium | Ge | II, IV | GeBr4, Ge(OH)2 |
| 33 | Arsenic/Arsenic | As | III, V | As2S5, H3AsO4 |
| 34 | Selenium | Se | II, IV, VI, | H2SeO3 |
| 35 | Bromine | Br | I, III, V, VII | HBrO3 |
| 36 | Krypton / Krypton | Kr | VI, IV, II | KrF2, BaKrO4 |
| 37 | Rubidium / Rubidium | Rb | I | RbH |
| 38 | Strontium / Strontium | Sr | II | SrSO4 |
| 39 | Yttrium / Yttrium | Y | III | Y2O3 |
| 40 | Zirconium / Zirconium | Zr | II, III, IV | ZrI4, ZrCl2 |
| 41 | Niobium / Niobium | Nb | I, II, III, IV, V | NbBr5 |
| 42 | Molybdenum | Mo | II, III, IV, V, VI | Mo2O5, MoF6 |
| 43 | Technetium / Technetium | Tc | I - VII | Tc2S7 |
| 44 | Ruthenium / Ruthenium | Ru | II - VIII | RuO4, RuF5, RuBr3 |
| 45 | Rhodium | Rh | I, II, III, IV, V | RhS, RhF3 |
| 46 | Palladium | Pd | I, II, III, IV | Pd2S, PdS2 |
| 47 | Silver | Ag | I, II, III | AgO, AgF2, AgNO3 |
| 48 | Cadmium | Cd | II | CdCl2 |
| 49 | Indium | In | III | In2O3 |
| 50 | Tin/Tin | Sn | II, IV | SnBr4, SnF2 |
| 51 | Antimony / Antimony | Sb | III, IV, V | SbF5, SbH3 |
| 52 | Tellurium / Tellurium | Te | VI, IV, II | TeH2, H6TeO6 |
| 53 | Iodine / Iodine | I | I, III, V, VII | HIO3,HI |
| 54 | Xenon / Xenon | Xe | II, IV, VI, VIII | XeF6, XeO4, XeF2 |
| 55 | Cesium | Cs | I | CsCl |
| 56 | Barium / Barium | Ba | II | Ba(OH)2 |
| 57 | Lanthanum / Lanthanum | La | III | LaH3 |
| 58 | Cerium | Ce | III, IV | CeO2, CeF3 |
| 59 | Praseodymium / Praseodymium | Pr | III, IV | PrF4, PrO2 |
| 60 | Neodymium / Neodymium | Nd | III | Nd2O3 |
| 61 | Promethium / Promethium | Pm | III | Pm2O3 |
| 62 | Samarium / Samarium | Sm | II, III | SmO |
| 63 | Europium | Eu | II, III | EuSO4 |
| 64 | Gadolinium / Gadolinium | Gd | III | GdCl3 |
| 65 | Terbium / Terbium | Tb | III, IV | TbF4, TbCl3 |
| 66 | Dysprosium / Dysprosium | Dy | III | Dy2O3 |
| 67 | Holmium | Ho | III | Ho2O3 |
| 68 | Erbium | Er | III | Er2O3 |
| 69 | Thulium | Tm | II, III | Tm2O3 |
| 70 | Ytterbium / Ytterbium | Yb | II, III | YO |
| 71 | Lutetium / Lutetium | Lu | III | LuF3 |
| 72 | Hafnium / Hafnium | Hf | II, III, IV | HfBr3, HfCl4 |
| 73 | Tantalum / Tantalum | Ta | I - V | TaCl5, TaBr2, TaCl4 |
| 74 | Tungsten/Tungsten | W | II - VI | WBr6, Na2WO4 |
| 75 | Rhenium / Rhenium | Re | I - VII | Re2S7, Re2O5 |
| 76 | Osmium / Osmium | Os | II - VI, VIII | OsF8, OsI2, Os2O3 |
| 77 | Iridium / Iridium | Ir | I - VI | IrS3, IrF4 |
| 78 | Platinum | Pt | I, II, III, IV, V | Pt(SO4)3, PtBr4 |
| 79 | Gold | Au | I, II, III | AuH, Au2O3, Au2Cl6 |
| 80 | Mercury | Hg | II | HgF2, HgBr2 |
| 81 | Thalium / Thallium | Tl | I, III | TlCl3, TlF |
| 82 | Lead/Lead | Pb | II, IV | PbS, PbH4 |
| 83 | Bismuth | Bi | III, V | BiF5, Bi2S3 |
| 84 | Polonium | Po | VI, IV, II | PoCl4, PoO3 |
| 85 | Astatine | At | no data | — |
| 86 | Radon / Radon | Rn | absent | — |
| 87 | Francium | Fr | I | — |
| 88 | Radium | Ra | II | RaBr2 |
| 89 | Actinium | Ac | III | AcCl3 |
| 90 | Thorium | Th | II, III, IV | ThO2, ThF4 |
| 91 | Proactinium / Protactinium | Pa | IV, V | PaCl5, PaF4 |
| 92 | Uranium / Uranium | U | III, IV | UF4, UO3 |
| 93 | Neptunium | Np | III - VI | NpF6, NpCl4 |
| 94 | Plutonium | Pu | II, III, IV | PuO2, PuF3, PuF4 |
| 95 | Americium | Am | III - VI | AmF3, AmO2 |
| 96 | Curium | Cm | III, IV | CmO2, Cm2O3 |
| 97 | Berkelium | Bk | III, IV | BkF3, BkO2 |
| 98 | Californium | Cf | II, III, IV | Cf2O3 |
| 99 | Einsteinium | Es | II, III | EsF3 |
| 100 | Fermium | Fm | II, III | — |
| 101 | Mendelevium | MD | II, III | — |
| 102 | Nobelium | No | II, III | — |
| 103 | Lawrence | Lr | III | — |
| Number | Element | Symbol | Valency of chemical elements | Example |
Crystallographic characteristics
system ; hexaoctahedral c. With. 3L44L36L29PC. Space group Fm3m (O5h). a0 = 3.9158. a0 = 3.924 A (pure Pt), 3.831 A (pure Ir), Z = 4
The crystal structure is close-packed cubic - atoms at the sites of a face-centered cubic lattice (Cu type).
The appearance of crystals. In combinations of faces, in addition to the dominant form {100}, {110}, {210}, {310} and some others are observed. Of the twins, twins of germination along (100) and fusion along (111) are predominantly developed. Polyxene crystals of skeletal development are known.
Application of platinum in the glass industry
Platinum is a metal, among other things, widely used in the production of high-quality optics. It is also often used in an alloy with rhodium in the manufacture of glass fiber dies, the thickness of which often does not exceed 1 micron. Such a metal can easily withstand thousands of hours of heating up to 1450 C. Also, the rhodium-platinum alloy practically does not react in any way to strong temperature changes and is resistant to corrosion.
Among other things, this metal is also very often used in the production of various types of equipment intended for the production of high-quality glass. Such mechanisms are not deformed or oxidized during the production process. They also do not react with the glass itself. Very often in this industry, platinum crucibles are used, for example. It is here that the widely known and very expensive Czech glass is made.
Form of being in nature
Usually observed in the form of small grains or nuggets weighing up to 8 kg. The largest nugget found in the primary deposits of the Urals weighed 427.5 g. Nuggets found in placers reached a size of 10 × 18 cm and a weight of 8–9 kg. The largest platinum nuggets displayed at the exhibition of the Diamond Fund of Russia weigh 5918.4 and 7860.5 grams. The rare small crystals are mostly cubic in shape.
Individual grains of native platinum found in ores are often grouped into small piles, sometimes forming continuous masses - nuggets.
Application in the production of nitric acid and other chemicals
In technology, platinum is used mainly as a catalyst. It is this metal that is the best oxidizer of ammonia to NO in the production of nitric acid. In this case, it is usually used in the form of a wire mesh with a diameter of 0.05-0.09 mm. In the production of nitric acid, it is most often not pure platinum that is used, but its alloy with rhodium. This allows the catalyst to be slightly cheaper, increases its activity and increases its shelf life.
Platinum is used in the technical industry, of course, not only in the production of nitric acid. Catalysts made from this metal can accelerate many other chemical reactions. Platinum is used, for example, in the hydrogenation of aromatic and technical hydrocarbons, ketones, acetylene, etc. This metal is also used in sulfuric acid production to produce SO3 or SO2.
Physical properties of platinum
Optical
- The color is steel gray to silver white with a yellow tint (platinum iridium) polyxene is silver white to steel black.
In polished sections, white, isotropic.
- The line is metallic, steel-gray.
- The shine is typical metallic.
Mechanical
Hardness is 4–4.5, for varieties rich in iridium – up to 6–7. There is no cleavage; the fracture is splintered, hooked. Ud. V. 21.5 (pure Pt); for platinum minerals less.—15–19. A connection between the reduced specific gravity and the presence of voids occupied by natural gases, as well as inclusions of foreign minerals, has been noted.
Other properties
Platinum iridium has a TV. 6-7, beats. V. 22.66, etc. 2360° S.
Chemical properties. Insoluble in acids (except aqua regia). T. pl. 1774° N.
Other properties . Pure platinum is non-magnetic, but such grains are extremely rare. Polyxene is magnetic, tetraferroplatinum is highly magnetic. Conducts electricity well. Very ductile (i.e. malleable and stretchy); for example, from 1 g of pure platinum you can draw a wire about 500 km long.
Diagnostic signs. In appearance, polyxene most closely resembles native silver and native iron. It differs from the first by its increased hardness, specific gravity and the fact that it does not melt under the blowing tube and does not dissolve in acids (except for aqua regia). Its insolubility in acids also distinguishes it from native iron.
Why is platinum so expensive?
Platinum is a premium metal, a marker of luxury.
This is natural:
- It is found on Earth 30 times less often than gold.
- To obtain 100 g of pure platinum raw materials, it is necessary to process 30 tons of ore. Gold is four times less.
- Only Russia, South Africa, the USA, China, and Zimbabwe have industrial-scale platinum deposits.
- World reserves are estimated in tons (not thousands of tons like gold).
World exchange prices for platinum exceed gold and also fluctuate depending on global market conditions. For example, at the end of December 2022, 1 gram costs 2.4 thousand rubles.
Origin
Found in primary deposits or placers. The former are of two types: a) inclusions or local impregnations of native platinum in ultrabasic igneous rocks (dunites) associated with chromite and b) copper-nickel sulfide accumulations in basic (norite) rocks. In basic magmas, at temperatures of 1300–1500° C, sulfides separate from the silicate melt. Chromite, osmium, iridium and platinum separate at higher temperatures; palladium is at the same or lower temperature and is thus captured in the sulfide melt. Therefore, native platinum and osmiride are the main minerals in the first type of deposits. The second type contains a lot of palladium and platinum, often found in the form of arsenide (sperrylite).
Contact-metamorphic platinum deposits, as well as quartz veins containing platinum, are also known.
Platinum group minerals are mostly found in typical igneous deposits genetically related to ultramafic igneous rocks. These minerals are among the last to be released in ore bodies (after silicates and oxides) at moments corresponding to the hydrothermal stage of the magmatic process.
Platinum minerals poor in palladium (polyxene, platinum iridium, etc.) are found in deposits among dunites - olivine feldspathic rocks rich in magnesia and poor in silica. Moreover, paragenetically they are extremely closely related to chrome spinels - oxides of complex composition: (Fe,Mg)(Cr,Al,Fe)2O4.
Palladium and nickel-palladide platinum are predominantly common in basic igneous rocks (norites, gabbro-norites) and are usually associated with sulfides: pyrrhotite (Fe1-XS), chalcopyrite (CuFeS2) and pentlandite - (Fe,Ni)9S8.
Under exogenous conditions, in the process of destruction of bedrock deposits and rocks, platinum placers are formed. Most of the minerals of the subgroup are chemically resistant under these conditions.
Receipt
Native platinum is mined at mines (see the article Noble Metals for more details); placer deposits of platinum are less rich, which are explored mainly by the method of spot testing.
The production of platinum in powder form began in 1805 by the English scientist W. H. Wollaston from South American ore.
Today, platinum is obtained from a concentrate of platinum metals. The concentrate is dissolved in aqua regia, after which ethanol and sugar syrup are added to remove excess HNO3. In this case, iridium and palladium are reduced to Ir3+ and Pd2+. The subsequent addition of ammonium chloride produces ammonium hexachloroplatinate (IV) (NH4)2PtCl6. The dried sediment is calcined at 800–1000 °C:
3(NH4)2[PtCl6] →T 2N2↑ + 2NH3↑ + 18HCl + 3Pt
The sponge platinum thus obtained is subjected to further purification by repeated dissolution in aqua regia, precipitation of (NH4)2PtCl6 and calcination of the residue. The purified sponge platinum is then melted into ingots. When reducing solutions of platinum salts by chemical or electrochemical methods, finely dispersed platinum is obtained - platinum black.
Place of Birth
Large deposits of the first type are known near Nizhny Tagil in the Urals. Here, in addition to primary deposits, there are also rich eluvial and alluvial placers. Examples of deposits of the second type are the Bushveld igneous complex in South Africa and Sudbury in Canada.
In the Urals, the first discoveries of native platinum that attracted attention date back to 1819. There it was discovered as an admixture to placer gold. Independently rich platinum placers, which are world famous, were discovered later. They are common in the Middle and Northern Urals and are all spatially confined to outcrops of massifs of ultrabasic rocks (dunites and pyroxenites). Numerous small primary deposits have been established in the Nizhny Tagil dunite massif. Accumulations of native platinum (polyxene) are confined mainly to chromite ore bodies, consisting mainly of chrome spinels with an admixture of silicates (olivine and serpentine). From the heterogeneous ultramafic Konder massif in the Khabarovsk Territory, platinum crystals of cubic habit, about 1–2 cm in size, come from the edge. A large amount of palladium platinum is mined from segregation sulfide copper-nickel ores of the Norilsk group deposits (North Central Siberia). Platinum can also be extracted from late magmatic titanomagnetite ores associated with the main rocks in such deposits as, for example, Gusevogorskoye and Kachkanarskoye (Middle Urals).
An analogue of Norilsk is of great importance in the platinum mining industry - the famous Sudbury deposit in Canada, from whose copper-nickel ores platinum metals are extracted along with nickel, copper and cobalt.
Where and how is it mined?
According to the theory, the process of formation of platinum is the same as that of gold - from interstellar dust as a result of a cosmic explosion.
She found herself in the core of the planet, then rose to the surface by earthquakes:
- It is found in igneous igneous rocks.
- Platinum veins are found in river beds, next to other minerals.
- Platinum in nature is usually found together with platinum group metals, iron, copper, and nickel. Most often these are large nuggets; cube crystals are rare.
Today the world leader in the extraction and supply of raw materials is South Africa. Russia is in the top five.
The methods for extracting platinum raw materials are similar to gold - washing and extraction from other ores. Then treatment using the refining method (removal of impurities) - double exposure to “regia vodka”, calcination.
Dissolving platinum in hot aqua regia
Practical use
During the first period of mining, native platinum did not find proper use and was even considered a harmful admixture to placer gold, with which it was caught along the way. At first, it was simply thrown into a dump when panning for gold or used instead of shot when shooting. Then attempts were made to falsify it by gilding it and handing it over to buyers in this form. Among the very first products made from Ural native platinum, stored in the St. Petersburg Mining Museum, were chains, rings, hoops for barrels, etc. The remarkable properties of platinum group metals were discovered somewhat later.
The main valuable properties of platinum metals are infusibility, electrical conductivity and chemical resistance. These properties determine the use of metals of this group in the chemical industry (for the manufacture of laboratory glassware, in the production of sulfuric acid, etc.), electrical engineering and other industries. Significant quantities of platinum are used in jewelry and dentistry. Platinum plays a critical role as a catalyst surface material in oil refining. The mined “raw” platinum is sent to refineries where complex chemical processes are carried out to separate it into its constituent pure metals.
Caring for platinum jewelry
Jewelry care includes:
- cleaning by a professional (especially if the jewelry contains precious stones);
- checking for good fixation of stones in the product;
- unlike you, jewelry does not benefit from cosmetics and household chemicals;
- inspect the jewelry for cracks, scratches, or chips. If you find damage, run to the jeweler.
Store jewelry in separate cases, boxes, bags. This way, products made from different metals and stones (with different hardnesses) will not scratch one another.
Platinum image of Lenin on the Order of Lenin.
Wash earrings and rings with a neutral soap solution using a soft brush. Place the chains in a glass, fill with soapy water and swirl the contents.
Then rinse the product under running water and dry.
Production
Platinum is one of the most expensive metals, its price is 3-4 times higher than gold, and about 100 times higher than silver
Platinum production is about 36 tons per year. The largest amounts of platinum are mined in Russia, South Africa, Kaiada, the USA and Colombia.
In Russia, platinum was first found in the Urals in the Verkh-Isetsky district in 1819. When washing gold-bearing rocks, white shiny grains were noticed in the gold, which did not dissolve even in strong acids. Bergprober of the laboratory of the St. Petersburg Mining Corps V.V. Lyubarsky examined these grains in 1823 and found that “the mysterious Siberian metal belongs to a special kind of raw platinum containing a significant amount of iridium and osmium.” In the same year, the highest order was issued to all mining chiefs to look for platinum, separate it from gold and present it to St. Petersburg. In 1824-1825, pure platinum placers were discovered in the Gorno-Blagodatsky and Nizhne-Tagil districts. And in the following years, platinum was found in several more places in the Urals. The Ural deposits were exceptionally rich and immediately brought Russia to first place in the world in the production of heavy white metal. In 1828, Russia mined an amount of platinum that was unheard of at that time - 1550 kg per year, about one and a half times more than was mined in South America for all the years from 1741 to 1825.
Interesting Facts
- The largest platinum nugget currently existing is the “Ural Giant” weighing 7,860.5. Kept in the Diamond Fund of the Moscow Kremlin.
- In South America in the 17th century, platinum was considered “fake silver” and one day its reserves were drowned in the ocean to prevent counterfeiting.
- The world's first platinum coins were issued in Russia (see Platinum coins).
- In Isaac Asimov's short story series "I, Robot" and his other works, the positronic brains of robots are made of spongy platinum (more precisely, an alloy of platinum and iridium).
Platinum. Stories and legends
Humanity has been familiar with platinum for more than two centuries. It was first noticed by members of the expedition of the French Academy of Sciences sent by the king to Peru. Don Antonio de Ulloa, a Spanish mathematician, was the first to mention it while on this expedition in his travel notes published in Madrid in 1748: “This metal has remained completely unknown since the beginning of the world until now, which is without a doubt very surprising.”
Platinum appears under the names “White Gold” and “Rotten Gold” in the literature of the 18th century. This metal has been known for a long time; its white, heavy grains were sometimes found during gold mining. It was assumed that it was not a special metal, but a mixture of two known metals. But they could not be processed in any way, and therefore platinum was not used for a long time. Until the 18th century, this most valuable metal, along with waste rock, was thrown into dumps. In the Urals and Siberia, grains of native platinum were used as shot for shooting. And in Europe, dishonest jewelers and counterfeiters were the first to use platinum.
In the second half of the 18th century, platinum was valued at half that of silver. It alloys well with gold and silver. Taking advantage of this, platinum began to be mixed with gold and silver, first in jewelry, and then in coins. Having learned about this, the Spanish government declared war on platinum “damage”. The Kopolevsky decree was issued, which ordered the destruction of all platinum mined along with gold. In accordance with this decree, officials of the mints in Santa Fe and Papayan (Spanish colonies in South America) solemnly, in front of numerous witnesses, periodically drowned the accumulated platinum in the Bogota and Cauca rivers. Only in 1778 this law was repealed, and the Spanish government itself began to mix platinum into gold coins. It is believed that the Englishman R. Watson was the first to obtain pure platinum in 1750. In 1752, after research by G. T. Schaeffer, it was recognized as a new metal. In the 70s of the 18th century, the first technical products from platinum (plates, wire, crucibles) were made.
What kind of jewelry is made from platinum?
Platinum is the most wear-resistant metal with anti-corrosion properties, representing a symbol of hardness and reliability. Imagine platinum wedding or engagement rings - timeless, like the deepest love.
photo of platinum jewelry
The big disadvantage of jewelry made from this alloy is its high cost; if we compare similar products made from white gold and platinum, the latter will be about three times more expensive. It is no coincidence that a comparison is made between these two metals, since they are very similar in appearance. It was the demand for white gold that gave rise to such popularity of platinum.
It is worth mentioning separately the combination of platinum and diamonds. Only such a gray-white frame can emphasize the depth and transparency of the stones. It’s not for nothing that before platinum and white gold became famous, diamonds were set in silver. And given the natural hardness of platinum, you will always know that the “stones” are always well secured in the ring. Such decoration can emphasize a high level of well-being and self-confidence.
Platinum Ingot
The jewelry industry is not the main one for this precious metal. In medicine, platinum is used to make electrodes for patients with angina pectoris, and is used in dentistry and in the treatment of cancer. It is also used to make mirrors for laser technology and to produce stable and durable electrical contacts.
Application in the chemical industry
In this case, platinum is also used mainly for the manufacture of crucibles and other laboratory equipment - cups, resistance thermometers, etc. Such products are used mainly in the manufacture of various kinds of ultrapure substances. In semiconductor crystals there should not be, for example, a single foreign atom per million of its own. These are the results that can be achieved using platinum equipment.
History of metal use
Platinum was known even before our era. It was used in Ancient Egypt to make a variety of jewelry. It was also common among the Incas, but was forgotten over time. In the photo you can see platinum items discovered by archaeologists:
Only after a long time, the discovery of this substance occurred thanks to Spanish travelers who were exploring South America. Initially, it was not appreciated, as the name suggests. "Platina" translated from Spanish can be formulated as "little silver." Accordingly, platinum was valued much less than precious metals. Often it was even considered unripe gold or incorrect silver (due to its color) and was simply thrown away. It is characterized by refractoriness and high density. Therefore it was considered unsuitable for any use.
However, then an interesting property was discovered - this precious metal has the ability to easily alloy with gold. Jewelers took this into account and actively began to mix platinum into gold products, thereby reducing the cost of their production. Moreover, this was done so skillfully that it was almost impossible to detect a fake. Due to the high density of platinum, even a small volume of it increased the weight of the finished product, but this was compensated by adding a certain amount of silver to the alloy, which did not affect the color. Such fraud was nevertheless recognized, and the import of the precious metal into Europe was prohibited by law for some time.
Platinum was recognized as an independent chemical element only in the mid-eighteenth century. A thorough study of its qualities made it possible to find the first use of this metal.
The physical and operational properties of platinum, especially resistance to various influences and high density, served as the basis for making useful equipment from it. In particular, platinum retorts were successfully used to concentrate caustic sulfuric acid.
Such vessels were initially made by forging or pressing, since in those days scientific progress could not provide the necessary temperature in furnaces for melting. By the end of the nineteenth century, it was possible to melt platinum using the flame generated by the combustion of detonating gas.
Jewelry area
More than 50 tons of platinum are used annually to make jewelry. Most of the products sold contain 95% pure mineral. Since there are few impurities in the accessories, they do not fade, do not lose their beautiful silver color and retain their shine for a long time. Platinum is used to create beautiful necklaces, chains, bracelets, earrings, and rings.
Jewelry with a pure composition does not irritate the skin, as is often the case with accessories containing allergenic metals. The bright shine resembles a diamond shine. Platinum serves as an excellent setting for precious stones. It is often combined with natural yellow tones of gold.
Platinum jewelry is often chosen due to its high durability. Jewelry accessories made of gold and silver wear out after some time, and they have to be sent in for repairs to replace the deteriorated layer with a new one. This does not happen with platinum items, so they serve their owners for a lifetime.