| Platinum | |
| Atomic number | 78 |
| Appearance of a simple substance | Heavy soft silver-white metal |
| Properties of the atom | |
| Atomic mass (molar mass) | 195.08 a. e.m. (/mol) |
| Atomic radius | 139 |
| Ionization energy (first electron) | 868.1(9.00) kJ/mol () |
| Electronic configuration | [Xe] 4f14 5d9 6s1 |
| Chemical properties | |
| Covalent radius | 130 |
| Ion radius | (+4e) 65 (+2e) 80 |
| Electronegativity (Pauling) | 2,28 |
| Electrode potential | Pt←Pt2+ 1.20V |
| Oxidation states | 4, 2, 0 |
| Thermodynamic properties of a simple substance | |
| Density | 21,45 /³ |
| Molar heat capacity | 25.85[1]/(mol) |
| Thermal conductivity | 71,6 /(·) |
| Melting temperature | 2045 |
| Heat of Melting | 21.76 kJ/mol |
| Boiling temperature | 4100 |
| Heat of vaporization | ~470 kJ/mol |
| Molar volume | 9.10 ³/mol |
| Crystal lattice of a simple substance | |
| Lattice structure | cubic face-centered |
| Lattice parameters | 3,920 |
| c/a ratio | n/a |
| Debye temperature | 230,00 |
| Pt | 78 |
| 195,08 | |
| 4f145d96s1 | |
| Platinum | |
Platinum - element 78 of the periodic table, atomic mass 195.08; noble metal of steel-gray color. Platinum was not known in the Old World, but the Andean civilizations (Inca and Chibcha) mined and used it since time immemorial. In Europe, platinum was unknown until the 18th century.
In 1735, the Spanish king issued a decree ordering that platinum should no longer be imported into Spain. When developing placers in Colombia, it was ordered to carefully separate it from the gold and drown it under the supervision of royal officials in the deep places of the Rio del Pinto River, which became known as the Platino del Pinto. And the platinum that had already been brought to Spain was ordered to be publicly and solemnly drowned in the sea. In 1748, the Spanish mathematician and navigator A. de Ulloa was the first to bring to the European continent samples of native platinum found in Peru. Platinum was first obtained in its pure form from ores by the English chemist W. Wollaston in 1803; the Italian chemist Gilius Scaliger discovered the indecomposability of platinum in 1835 and thus proved that it is an independent chemical element. In Russia, back in 1819, a “new Siberian metal” was discovered in alluvial gold mined in the Urals. At first it was called white gold; platinum was found at the Verkh-Isetsky, and then at the Nevyansky and Bilimbaevsky mines. Rich placers of platinum were discovered in the second half of 1824, and the following year its mining began in Russia.
origin of name
The name platinum was given by the Spanish conquistadors, who in the middle of the 16th century. first became acquainted in South America (on the territory of modern Colombia) with a new metal that looked like silver ( plata
).
Spanish word Platina
literally means “little silver”, “little silver” (platinum versus silver was half the price). This disparaging name is explained by the exceptional refractoriness of platinum, which could not be melted down, was not used for a long time and was valued half as much as silver.
Platinum crystal lattice:
| 500 | Crystal cell | |
| 511 | Crystal grid #1 | |
| 512 | Lattice structure | Cubic face centered |
| 513 | Lattice parameters | 3.920 Å |
| 514 | c/a ratio | |
| 515 | Debye temperature | 230 K |
| 516 | Name of space symmetry group | Fm_3m |
| 517 | Symmetry space group number | 225 |
Receipt
Native platinum is mined in mines (see more in the article Noble metals)
The production of platinum in powder form began in 1805 by the English scientist W. H. Wollaston from South American ore. Today, platinum is obtained from a concentrate of platinum metals. The concentrate is dissolved in aqua regia, after which ethanol and sugar syrup are added to remove excess HNO3. In this case, iridium and palladium are reduced to Ir3+ and Pd2+. Subsequent addition of ammonium chloride produces (NH4)2PtCl6. The dried sediment is calcined at 800-1000°C: (NH4)2PtCl6 = N2 + 6HCl + Pt + H2. The sponge platinum thus obtained is subjected to further purification by repeated dissolution in aqua regia, precipitation of (NH4)2PtCl6 and calcination of the residue. The purified sponge platinum is then melted into ingots. When platinum solutions are reduced by chemical or electrochemical methods, finely dispersed platinum is obtained - platinum black.
Chemical properties
Platinum's chemical properties are similar to palladium, but it exhibits greater chemical stability. Reacts only with hot aqua regia: 3Pt + 4HNO3 + 18HCl = 3H2[PtCl6] + 4NO + 8H2O
Platinum dissolves slowly in hot sulfuric acid and liquid bromine. It does not interact with other mineral and organic acids. When heated, it reacts with alkalis and sodium peroxide, halogens (especially in the presence of alkali metal halides): Pt + 2Cl2 + 2NaCl = Na2[PtCl6]. When heated, platinum reacts with sulfur, selenium, tellurium, carbon and silicon. Like palladium, platinum can dissolve molecular hydrogen, but the volume of absorbed hydrogen is smaller and the ability of platinum to release it when heated is less.
When heated, platinum reacts with oxygen to form volatile oxides. The following platinum oxides are identified: black PtO, brown PtO2, reddish-brown PtO3, as well as Pt2O3 and Pt3O4.
The hydroxides Pt(OH)2 and Pt(OH)4 are known for platinum. They are obtained by alkaline hydrolysis of the corresponding chloroplatinates, for example: Na2PtCl4 + 2NaOH = 4NaCl + Pt(OH)2I, Na2PtCl6 + 4NaOH = 6NaCl + Pt(OH)4I. These hydroxides exhibit amphoteric properties: Pt(OH)2 + 2NaOH = Na2[Pt(OH)4], Pt(OH)2 + 4HCl = H2[PtCl4] + 2H2O, Pt(OH)4 + 6HCl = H2[PtCl6] + 4H2O, Pt(OH)4 + 2NaOH = Na2[Pt(OH)6]. PtF6 hexafluoride is one of the strongest oxidizing agents, capable of oxidizing oxygen, xenon or NO molecules: O2 + PtF6 = O2+[PtF6]-.
With the interaction between Xe and PtF6 discovered by N. Bartlett, leading to the formation of XePtF6, the chemistry of inert gases began. PtF6 is obtained by fluorinating platinum at 1000 °C under pressure. Fluoridation of platinum at normal pressure and temperature 350-400 °C gives Pt(IV) fluoride: Pt + 2F2 = PtF4 Platinum fluorides are hygroscopic and decompose with water. Platinum(IV) tetrachloride with water forms hydrates PtCl4·nH2O, where n = 1, 4, 5 and 7. By dissolving PtCl4 in hydrochloric acid, chloroplatinic acids H[PtCl5] and H2[PtCl6] are obtained. Platinum halides such as PtBr4, PtCl2, PtCl2·2PtCl3, PtBr2 and PtI2 have been synthesized. Platinum is characterized by the formation of complex compounds of the composition [PtX4]2- and [PtX6]2-. While studying platinum complexes, A. Werner formulated the theory of complex compounds and explained the nature of the occurrence of isomers in complex compounds.
Reactivity
Coin 3 rubles, 1834
Platinum is one of the most inert metals. It is insoluble in acids and alkalis, with the exception of aqua regia. Platinum also reacts directly with bromine, dissolving in it.
When heated, platinum becomes more reactive. It reacts with peroxides, and upon contact with atmospheric oxygen, with alkalis. A thin platinum wire burns in fluorine, releasing a large amount of heat. Reactions with other non-metals (chlorine, sulfur, phosphorus) occur less readily. When heated more strongly, platinum reacts with carbon and silicon, forming solid solutions, similar to the iron group metals.
In its compounds, platinum exhibits almost all oxidation states from 0 to +8, of which +2 and +4 are the most stable. Platinum is characterized by the formation of numerous complex compounds, of which many hundreds are known. Many of them bear the names of the chemists who studied them (salts of Cossus, Magnus, Peirone, Zeise, Chugaev, etc.). A great contribution to the study of such compounds was made by the Russian chemist L. A. Chugaev (1873−1922), the first director of the Institute for the Study of Platinum, created in 1918.
Platinum hexafluoride PtF6 is one of the strongest oxidizing agents among all known chemical compounds. With its help, in particular, Canadian chemist Neil Bartlett in 1962 obtained the first true chemical compound of xenon, XePtF6.
Catalyst
Platinum, especially in a finely dispersed state, is a very active catalyst for many chemical reactions, including those used on an industrial scale. For example, platinum catalyzes the reaction of hydrogen addition to aromatic compounds even at room temperature and atmospheric pressure of hydrogen. Back in 1821, the German chemist I. V. Döbereiner discovered that platinum black promotes the occurrence of a number of chemical reactions; however, the platinum itself did not undergo changes. Thus, platinum black oxidized vapors of wine alcohol to acetic acid already at ordinary temperatures. Two years later, Döbereiner discovered the ability of spongy platinum to ignite hydrogen at room temperature. If a mixture of hydrogen and oxygen (explosive gas) is brought into contact with platinum black or spongy platinum, then at first a relatively calm combustion reaction occurs. But since this reaction is accompanied by the release of a large amount of heat, the platinum sponge becomes hot and the explosive gas explodes. Based on his discovery, Döbereiner designed the “hydrogen flint,” a device that was widely used to produce fire before the invention of matches.
Production
Until 1748, platinum was mined and produced only in America and was not known in the Old World.
When platinum began to be imported to Europe, its price was half that of silver. Jewelers very quickly discovered that platinum alloys well with gold, and since the density of platinum is higher than that of gold, minor additions of silver made it possible to produce fakes that could not be distinguished from gold items. This kind of counterfeit became so widespread that the Spanish king ordered the import of platinum to be stopped and the remaining reserves to be drowned in the sea. This law lasted until 1778. After the law was repealed, there was little demand for platinum; it was used mainly to create chemical equipment, devices and as catalysts. The platinum mined in America was sufficient for these purposes. There is no significant industrial production to speak of.
In 1819, platinum was first discovered in the Urals near Yekaterinburg, and in 1824 platinum placers were discovered in the Nizhny Tagil district. The explored reserves of platinum were so large that Russia almost immediately took first place in the world in the extraction of this metal. In 1828 alone, 1.5 tons of platinum were mined in Russia - more than in 100 years in South America. By the end of the 19th century, platinum was mined in Russia 40 times more than in all other countries of the world. Moreover, it was also represented by very weighty nuggets. For example, one of the nuggets found in the Urals weighed 9.6 kg.
By the middle of the 19th century. Extensive research on platinum refining was carried out in England and France. In 1859, French chemist Henri Etienne Sainte-Clair Deville first developed an industrial method for producing pure platinum ingots. From that time on, almost all the platinum mined in the Urals was bought up by English and French companies, in particular Johnson, Matthay and Co. Later, American and German companies joined in purchasing platinum from Russia.
Even after significant foreign purchases, most of the platinum mined by Russia did not find worthy use. Therefore, starting in 1828, at the proposal of the Minister of Finance Yegor Kankrin, platinum coins in denominations of 3.6 and 12 rubles began to be issued in Russia. At the same time, the 12-ruble platinum coin had a mass of 41.41 g, and the ruble silver coin contained 18 g of pure silver. That is, in terms of metal value, platinum coins were 5.2 times more expensive than silver coins. From 1828 to 1845 1,372,000 three-ruble coins, 17,582 six-ruble and 3,303 twelve-ruble coins with a total weight of 14.7 tons were issued. The main benefit from the mining was received by the owners of the mines - the Demidovs. Estimate: in 1840 alone, 3.4 tons of platinum were mined. In 1845, at the insistence of the Minister of Finance Fyodor Vronchenko, the production of platinum coins was stopped and all of them were urgently withdrawn from circulation. The main version of such a hasty step is considered to be an increase in European prices for platinum, as a result of which the coins became more expensive than their face value. After the cessation of coinage, platinum production in Russia fell 20 times and by 1915 Russia accounted for only 95% of world platinum production. The remaining 5% was produced by Colombia. Moreover, almost all Russian platinum was exported. For example, in 1867, England bought up the entire reserve of Russian platinum - more than 16 tons.
By the end of the 19th century. Russia produced 4.5 tons of platinum per year.
Before World War I, Colombia was the second largest platinum-producing country after Russia; since the 1930s became Canada, and after World War II - South Africa.
In 1952, Colombia produced 0.75 tons of platinum, the USA - 0.88 tons, Canada - 3.75 tons, and the Union of South Africa - 7.2 tons. In the USSR, data on platinum production was classified.
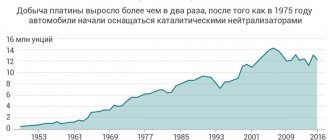
In 2007, 213 tons of platinum were mined in the world, and in 2008 - 200 tons. The leaders in production were: South Africa (166.0 tons were mined in 2007, and 153.0 tons in 2008), Russia (27.0 tons). /25.0), Canada (6.2/7.2), Zimbabwe (5.3/5.6), USA (3.9/3.7), Colombia (1.4/1.7). [3]
The leader in platinum production in Russia is MMC Norilsk Nickel.
The world's proven reserves of platinum group metals amount to about 80,000 tons and are distributed mainly between South Africa (87.5%), Russia (8.3%) and the USA (2.5%).
Electronic structure of the silver atom
The silver atom consists of a positively charged nucleus (+47), inside of which there are 47 protons and 61 neutrons, and 42\7 electrons move around in five orbits.
Fig.1. Schematic structure of the silver atom.
The distribution of electrons among orbitals is as follows:
The valence electrons of a silver atom are considered to be electrons located in the 4d and 5s orbitals. The energy diagram of the ground state takes the following form:
The valence electrons of a silver atom can be characterized by a set of four quantum numbers: n (principal quantum), l (orbital), ml (magnetic) and s (spin):
Application
In technology
- Since the first quarter of the 19th century, it has been used in Russia as an alloying additive for the production of high-strength steels[4]
- Platinum is used as a catalyst (most often in an alloy with rhodium, and also in the form of platinum black
- a fine powder of platinum obtained by reducing its compounds). - Platinum is used in jewelry, dentistry, and medicine.
- Production of chemically and heat-resistant laboratory glassware.
- Production of miniature magnets of enormous strength (platinum-cobalt alloy, PlK-78).
- Special mirrors for laser technology.
- Extremely durable and stable electrical contacts and alloys for radio engineering (Pli-10, Pli-20, Pli-30 (platinum-iridium).
- Galvanic coatings.
- Distillation retorts for the production of hydrofluoric acid.
- Electrodes for the production of perchlorates, perborates, percarbonates, peroxodisulfuric acid (in fact, the entire world production of hydrogen peroxide is based on platinum: electrolysis of sulfuric acid - peroxodisulfuric acid - hydrolysis - distillation of hydrogen peroxide).
- Insoluble anodes in electroplating.
- Anode rods for corrosion protection of submarine hulls.
- Heating elements of resistance furnaces.
In medicine
Platinum compounds (mainly tetrachloroplatinates) are used as cytostatics (“cis-platinum”). However, more effective anticancer drugs are now available.
In jewelry
Platinum and its alloys are widely used in jewelry production.
Every year the global jewelry industry consumes about 50 tons of platinum. Before 2001, most platinum jewelry was consumed in Japan. Since 2001, China has accounted for approximately 50% of global sales. In 1980, China consumed about 1% of platinum jewelry. Currently, about 10 million platinum products with a total weight of about 25 tons are sold annually in China.
Russian demand for jewelry platinum is 0.1% of the world level.
Monetary function
Main article
:
Platinum coins
Platinum coin of 1835 with a face value of 12 rubles.
Platinum, gold and silver are the main metals that perform a monetary function. However, platinum began to be used for making coins several thousand years later than gold and silver.
The world's first platinum coins were issued and circulated in the Russian Empire from 1828 to 1845. Minting began with three-ruble coins. In 1829, “platinum duplons” (six-ruble coins) were established, and in 1830, “quadruples” (twelve-ruble coins). The following coin denominations were minted: 3, 6 and 12 rubles. 1,371,691 pieces of three-ruble coins were minted, 14,847 pieces of six-ruble coins were minted. and twelve-ruble coins - 3474 pcs.[2]
In 1846, the minting of platinum coins was stopped, although by this year the production of Ural platinum amounted to about 2,000 poods or 32,000 kg, of which 14,669 kg were minted into coins. A huge amount of platinum, accumulated at the St. Petersburg Mint, partly in the form of coins, and partly in unprocessed form (according to various sources, from 720 to 2000 poods), was sold to the English company Johnson, Matte and Co. As a result, England, which did not mine a single gram of platinum, had a monopolist in this industry for a long time.[5]
After 1846, no country allowed itself the “luxury” of introducing platinum coins into circulation. Platinum coins currently issued by different countries are bullion coins. In the period from 1992 to 1995, investment platinum coins in denominations of 25, 50 and 150 rubles were issued by the Bank of Russia.
How to determine authenticity
They offer silver or white gold under the guise of platinum.
You can determine the authenticity of a product at home:
- A product made of platinum is heavier than gold, especially silver of comparable parameters.
- Place the silver item and the sample next to it. Even high-grade silver gives off a grayish tint and “coldness.” The platinum composition has a pure warm white luster.
There are other differences between platinum and white gold:
- Platinum rings, earrings, even necklaces and bracelets are not large or massive - the metal is too expensive.
- At normal temperatures, it is not affected by hydrogen peroxide, iodine solution, or acetic acid. They will not leave marks on the surface.
- Under the flame of a candle, lighter, or household gas stove, it will heat up, but will not change in appearance. Gold may soften.
- You can hold the sample in your hands: the royal metal takes longer to heat up than gold, especially silver.
- A pawn shop worker or jeweler is guaranteed to be able to distinguish the original.
Platinum looks almost like white gold, so many are sure that they are the same thing. However, white gold is an alloy of metals, and platinum is an independent element.
Interesting Facts
- The largest platinum nugget currently existing is the “Ural Giant” weighing 7,860.5. Kept in the Diamond Fund of the Moscow Kremlin.
- In South America in the 17th century, platinum was considered “fake silver” and one day its reserves were drowned in the ocean to prevent counterfeiting.
- The world's first platinum coins were issued in Russia (see Platinum coins).
- In Isaac Asimov's short story series "I, Robot" and his other works, the positronic brains of robots are made of spongy platinum (more precisely, an alloy of platinum and iridium).
How to store and care
In order for platinum products to maintain their original premium condition, simple care is sufficient:
- Jewelry (especially rings and bracelets) is removed before household work (washing, cleaning, cooking).
- They are protected from moisture and household chemicals, especially aggressive ones.
- It should be stored in a separate, tightly closed box.
Tarnish or dirt can be removed by immersing the item in a warm soapy solution for a couple of hours. Then rinse with water and dry with a soft cloth.
If possible, purchase a cleaning product for platinum products.
If the jewelry contains diamonds or other stones, it should be sent for professional cleaning.