Jewelers call platinum the queen of precious metals. But it was not always so. Until the 18th century, it was not mined on an industrial scale, and was even called “bad silver.” Let's figure out what platinum is and what its value is. We will learn many interesting facts about what it looks like in its original form, how it is mined and used.
If you are the happy owner of platinum jewelry or just want to become one, let’s find out how to “not fall for” a fake.
What is platinum
This is a silver-colored precious metal. Outwardly it resembles silver, but has completely different physical and chemical properties.
Platinum deposits are rarely found in nature. Their development is extremely labor-intensive. For these reasons, the price of the metal on the market is even higher than gold.
Expert opinion
Vsevolod Kozlovsky
6 years in jewelry making. Knows everything about samples and can identify a fake in 12 seconds
Platinum (as denoted in chemistry, Pt, platinum) is one of the elements of the periodic table. This is a dense, hard, but very plastic material.
Brief history of appearance
The ancient Egyptians, Incas and Chibcha tribes used the metal as jewelry. Platinum came to the European continent along with Spanish sailors from South America. The precious metal was not appreciated at that time. Even the word platina translated from Spanish sounds like “dirty silver.” Since it has a high density and is refractory, it was considered unsuitable for consumption. Often they even threw it away.
Fraudsters were the first to use Pt in jewelry. It was added to gold alloys, increasing the weight of the item without reducing the cost. Production reached such a scale that the import of platinum into Europe was banned.
Only in the middle of the 18th century the metal was isolated as a separate chemical element. Properties were studied - operational, physical. At the turn of the 19th century, scientists discovered that platinum is not just a noble metal, but also serves as the “mother” for a whole family of platinum group metals:
- palladium;
- rhodium;
- osmia;
- iridium.
origin of name
The name platinum was given by the Spanish conquistadors, who in the middle of the 16th century. first became acquainted in South America (on the territory of modern Colombia) with a new metal that looked like silver ( plata
).
Spanish word Platina
literally means “little silver”, “little silver” (platinum versus silver was half the price). This disparaging name is explained by the exceptional refractoriness of platinum, which could not be melted down, was not used for a long time and was valued half as much as silver.
What does platinum look like in nature?
It is not found in its pure form in natural conditions. Forms isomorphic mixtures with iron, copper, silver, nickel, and platinum group metals. Platinum-containing ore has small grains and inclusions of precious metal.
Native metal is considered to be mined nuggets containing from 75 to 92% Pt. They are rarely found. Ferrous platinum (polyxene), which contains 20-50% iron, is mainly mined.
The process of formation in nature
Platinum ores are in a dispersed state. They are of igneous origin, released through the crystallization of basic and ultrabasic magmas. At a temperature of 1300-1500 degrees, sulfides, platinum, chloride, osmium, and iridium are separated from the silicate melt.
The surface of bedrock deposits is destroyed over time. The resulting placers are convenient for industrial development.
Structure, chemical and physical properties
Expert opinion
Vsevolod Kozlovsky
6 years in jewelry making. Knows everything about samples and can identify a fake in 12 seconds
The structural structure of the crystal lattice is a cube with third-order symmetry elements. The metal has refractoriness, low thermal conductivity, and high density (21.45 g per dm2). Melting point - 1769 degrees, boiling point - 3800.
Hard material is difficult to process. It is so durable that jewelry can be made from pure platinum without adding impurities.
Other physical and chemical properties:
- plasticity when heated (you can make the thinnest foil or wire);
- resistance to corrosion, oxidation;
- lack of interaction with acids and alkalis;
- low resistivity (serves as a good conductor);
- catalyst for many chemical reactions.
Also watch the video for other properties of platinum:
PROPERTIES
The color of polyxene ranges from silver-white to steel-black. The dash is metallic steel gray. The shine is typical metallic. The reflectivity in polished sections is high - 65-70. Hardness is 4-4.5, for varieties rich in iridium – up to 6-7. It has malleability. The fracture is hooked. Cleavage is usually absent. Ud. weight 15-19. A connection between the reduced specific gravity and the presence of voids occupied by natural gases, as well as inclusions of foreign minerals, has been noted. It is magnetic and paramagnetic. Conducts electricity well. Platinum is one of the most inert metals. It is insoluble in acids and alkalis, with the exception of aqua regia. Platinum also reacts directly with bromine, dissolving in it.
When heated, platinum becomes more reactive. It reacts with peroxides, and upon contact with atmospheric oxygen, with alkalis. A thin platinum wire burns in fluorine, releasing a large amount of heat. Reactions with other non-metals (chlorine, sulfur, phosphorus) occur less actively. When heated more strongly, platinum reacts with carbon and silicon, forming solid solutions, similar to the iron group metals.
How are platinum veins found?
The main place of production is copper and nickel deposits (primary and alluvial). From them, platinum is mined along with other metals. Platinum nuggets are found in ultramafic igneous rocks. Natural mineral ores with high element content are rare.
Satellites of platinum
In deposits, platinum accompanies platinum group metals.
In addition, in various rocks, platinum is found with the following accompanying minerals:
- Basic and ultrabasic magmas—serpentine, chromite, magnetite, chrysotile-asbestos, olivine, orthorhombic pyroxenes.
- Placers - chromite, corundum, magnetite, gold, diamonds.
- Diabase - chalcopyrite.
Where is platinum found in nature?
This is the rarest of the elements of the Earth's crust.
Found as nuggets, alloys with nickel, copper, and platinum group metals.
Deposits where platinum is found are associated with mafic and ultramafic igneous rocks.
Receipt
Native platinum is mined in mines (see more in the article Noble metals)
The production of platinum in powder form began in 1805 by the English scientist W. H. Wollaston from South American ore. Today, platinum is obtained from a concentrate of platinum metals. The concentrate is dissolved in aqua regia, after which ethanol and sugar syrup are added to remove excess HNO3. In this case, iridium and palladium are reduced to Ir3+ and Pd2+. Subsequent addition of ammonium chloride produces (NH4)2PtCl6. The dried sediment is calcined at 800-1000°C: (NH4)2PtCl6 = N2 + 6HCl + Pt + H2. The sponge platinum thus obtained is subjected to further purification by repeated dissolution in aqua regia, precipitation of (NH4)2PtCl6 and calcination of the residue. The purified sponge platinum is then melted into ingots. When platinum solutions are reduced by chemical or electrochemical methods, finely dispersed platinum is obtained - platinum black.
Top countries by production
The leading countries in the world market are:
- Republic of South Africa.
- Russia.
- Zimbabwe.
- USA.
- Canada.
World platinum reserves
80% of platinum group metal deposits available for development are located in South Africa (Bushveld complex in the north of the country).
The second largest reserves are the GreatDyke field (Zimbabwe).
3rd and 4th place in the Russian Federation and North America (USA, Canada). Canadian platinum ores are concentrated in the provinces of Ontario and Manitoba. In the United States, the bulk of production occurs in two large mines in Montana.
Colombian placer deposits are saturated with platinum group metals. They are concentrated in the western Cordillera, in the river valleys of Atrato and San Juan.
Crystallographic characteristics
system ; hexaoctahedral c. With. 3L44L36L29PC. Space group Fm3m (O5h). a0 = 3.9158. a0 = 3.924 A (pure Pt), 3.831 A (pure Ir), Z = 4
The crystal structure is close-packed cubic - atoms at the sites of a face-centered cubic lattice (Cu type).
The appearance of crystals. In combinations of faces, in addition to the dominant form {100}, {110}, {210}, {310} and some others are observed. Of the twins, twins of germination along (100) and fusion along (111) are predominantly developed. Polyxene crystals of skeletal development are known.
Extraction methods
The production process consists of three stages:
- Ore mining.
- Enrichment.
- Obtaining precious metal of high purity.
Extracting platinum from the earth's interior is a labor-intensive and expensive task. To extract 1 ounce (31.1 grams) of precious metal, more than 10 tons of ore are processed.
There are two ways to get it:
- open;
- underground.
The first option is suitable for placer deposits formed as a result of the destruction of primary rock. It involves the use of quarry equipment, dredges, and hydromechanical means.
Primary deposits and buried placers are mined underground. Shafts are dug, holes are manually drilled into them, and explosives are placed. Miners lift pieces of rock that break off to the surface for further processing. Today, this version of metal mining is significantly mechanized, but it cannot be done without manual labor.
Areas of application
The industries that use platinum are varied.
List of applications:
- oil and gas industry (production of high-octane gasoline and technical hydrogen from oil fractions);
- automotive industry (production of exhaust gas afterburning catalysts);
- electrical engineering (elements of high-temperature furnaces, mirrors for lasers, magnets);
- ammonia synthesis;
- chemical, glass industry (equipment with high resistance to chemical and temperature influences, electrodes, reaction catalysts);
- medical instruments;
- making jewelry.
The richest platinum deposits
Total world reserves in known deposits are about 66 thousand tons. Most of them are located in South Africa (63 thousand tons). Russian deposits are rich in 1.1 thousand tons, American - 0.9 thousand tons, Canadian - 0.3 thousand tons, other countries - 0.7 thousand tons.
In the world
The largest deposits of platinum-containing ores are located in South Africa. These are ultramafic rocks from the Paleozoic era at Bushwell.
Other major world deposits:
- Sudbury (Canada);
- Nevada, California, Wyoming, Alaska (USA);
- Quibdo, Andagoda, Opogodo, Tamana, Condoto-Iro (Colombia);
- Norway, New Zealand.
In Russia
For the first time on the territory of the Russian Federation, deposits were discovered in the 20s of the 19th century in the Ural Verkh-Isetsky district.
Main platinum deposits:
- Oktyabrskoe;
- Talnakhskoe;
- Nizhny Tagil;
- sulfide-copper-nickel in Norilsk, Krasnodar region, Fedorova tundra, Zarechenskoye in the Murmansk region;
- alluvial deposits in the Khabarovsk Territory (Konder), in Kamchatka (Levtyrinyvayam), on the Lobva River, Vyysko-Isovskoye.
Story
Platinum was not known in the Old World until the mid-16th century, but the Andean civilizations (Inca and Chibcha) have mined and used it since time immemorial. The first Europeans to become acquainted with platinum in the middle of the 16th century were the conquistadors. It is believed that Scaliger was the first to mention platinum in literature in the book “Exoteric Exercises in 15 Books” published in 1557, where he, arguing with Cardano about the concept of “metal”, spoke about a certain substance from Honduras that cannot be melted. This substance was probably platinum.
In 1735, the Spanish king issued a decree ordering that platinum should no longer be imported into Spain. When developing placers in Colombia, it was ordered to carefully separate it from the gold and drown it under the supervision of royal officials in the deep places of the Rio del Pinto River (a tributary of the Rio San Juan (English) Russian), which became known as the Platino del Pinto. And the platinum that had already been brought to Spain was ordered to be publicly and solemnly drowned in the sea. The royal order was reversed 40 years later when the Madrid authorities ordered platinum to be delivered to Spain in order to counterfeit gold and silver coins themselves. In 1820, between 3 and 7 tons of platinum were delivered to Europe. Here alchemists met her, who considered gold to be the heaviest metal. The unusually dense platinum turned out to be heavier than gold, so alchemists considered it an unsuitable metal and endowed it with hellish traits. Platinum found some use later in France, when the meter standard was made from it, and later the kilogram standard.
According to some sources, the Spanish mathematician and navigator A. de Ulloa brought samples of platinum to London in 1744; he included a description of platinum in his report on a trip to South America, published in 1748. In 1789, A. Lavoisier included platinum in the list of simple substances. Platinum was first obtained in its pure form from ores by the English chemist W. Wollaston in 1803.
In Russia, back in 1819, a “new Siberian metal” was discovered in alluvial gold mined in the Urals, which was first called white gold. Platinum was found at the Verkh-Isetsky, and then at the Nevyansk and Bilimbaevsky mines. Rich placers of platinum were discovered in the second half of 1824, and the following year its mining began in Russia. In 1826, P. G. Sobolevsky and V. V. Lyubarsky invented a method for producing malleable platinum using pressing and subsequent exposure to a white-hot state.
Advantages and disadvantages
The benefits of platinum are due to its physical and chemical properties. Among them are hardness, low thermal conductivity, high density, and fire resistance. The metal does not deform when heated and is resistant to corrosion. It is almost impossible to bend or deform.
Platinum jewelry is hypoallergenic, wear-resistant, and durable.
The durability and strength of the precious metal is evidenced by the fact that kilogram and meter standards were made from it at the end of the 18th century.
Against the background of all the listed advantages, there is only one drawback. This is the price. The cost of platinum products is significantly higher than gold ones.
PHYSICAL PROPERTIES
| Mineral color | steel gray fading to dark gray |
| Stroke color | gray-white |
| Transparency | opaque |
| Shine | metal |
| Cleavage | No |
| Hardness (Mohs scale) | 4-4,5 |
| Strength | malleable |
| Kink | serrated, hooked |
| Density (measured) | 14 – 19 g/cm3 |
| Radioactivity (GRapi) | 0 |
| Magnetism | paramagnetic |
Kinds
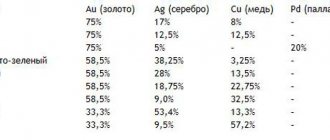
Native platinum is classified depending on the content of other components in the ore. There is palladium platinum (40% palladium), nickel (3% nickel), rhodium (5% rhodium), cuproplatinum (10-15% copper), ferruginous (25% nickel and iron each).
Measured, stamped 999 fine ingots are produced from enriched pure platinum. Due to its high cost, Pt jewelry is usually small and monolithic.
Alloys and samples
Platinum alloys, like the pure element, are grayish-white with a characteristic shine.
The list indicates what the ligature consists of. For the alloy, one or more of the following components are taken:
- copper;
- rhodium;
- palladium;
- gold;
- cobalt;
- iridium.
Products made from precious metals, including platinum, are subject to mandatory testing. Placing a state stamp confirms the conformity of the alloy to a particular sample.
According to the metric system adopted in Russia, 850, 900, 950, 999 samples are approved for platinum. The stamp is a rectangle with beveled edges, which depicts the profile of a woman in a kokoshnik and a digital designation of the sample.
Sample correspondence table
The metric system shows how many units of pure precious metal are in 1000 units of the alloy. The carat standard is based on pure metal as 24 units. The formula for converting a metric sample into a carat sample is: sample × 24/100.
| Metric | Carat |
| 850 | 20 |
| 900 | 22 |
| 950 | 23 |
| 999 | 24 |
Conclusion
Most people think of platinum as a very expensive, silvery-white metal that is used to make jewelry. However, due to its numerous properties, it has become widespread in various fields of human activity, from medicine to the automotive industry.
Although platinum has never been used as money in its history, investing in platinum is considered a fairly profitable investment. One ounce of this metal exceeds the value of a similar amount of gold by $270. If you constantly monitor the exchange rate of precious metals, you can make a good profit.
Where can you buy or sell
Demand on the platinum sales market so far exceeds its supply. This is due to the complex extraction from the subsoil. High purity platinum bars and coins can be purchased in banks. They can also be bought back there (usually with a certificate and receipt). Platinum jewelry, which is made by famous jewelry houses, cannot be found in an ordinary store.
You can sell platinum jewelry or scrap at a pawnshop or auction house. The price there will not be the best. The advantage is that the money will be given out immediately. Thrift stores can sell items at a better price, but this may not take a single day. Money will be given to the seller only after the sale of the goods.
You can get a favorable price when selling at auctions, including on the Internet. But an inexperienced seller, in pursuit of profit, can run into scammers.
Expert opinion
Lyudmila Pestereva
Our most experienced gold investor
Ask a Question
I advise you to find collectors. They will evaluate the value of the product not only in grams, but also taking into account its artistic, historical, and jewelry value. In this case, you can sell the jewelry at a price as close as possible to the market price.
How much does 1 gram cost today?
Prices per ounce of precious metal are set twice a day on the London Stock Exchange. The figure is taken as a basis by the central banks of the world's countries, including the Central Bank of the Russian Federation. Below is a live chart and table with current platinum prices.
Platinum | RUB | 1 Gram
USDRUB*PL1!*10000000/311034768 chart provided by TradingView
Platinum | USD | 1 Gram
Chart PL1!*10000000/311034768 courtesy of TradingView
Price per scrap
All items accepted at the pawnshop are assessed as scrap. The cost of platinum scrap is, of course, lower than the market price - by about 15-20%. Some pawn shops value jewelry by category. For high-quality items, a large amount is given per gram.
How to spot a fake
Platinum's silvery luster is similar to silver. Let's figure out how to avoid scammers and distinguish real platinum jewelry from a fake.
The element does not react to alkalis and acids. There is a simple home test using a rotten egg, which contains hydrogen sulfide. By what color the product becomes, you can determine what is in front of you. Silver will turn black, platinum will not.
The old-fashioned method of biting will help determine how soft the metal is in front of you. Platinum alloys are very hard and there are no traces of deformation on them. Of course, not every buyer will risk their teeth. And sellers are not happy with this definition of authenticity.
A distinctive characteristic of platinum is low thermal conductivity. When heated, heat spreads more slowly than with other metals.
I advise you to pay attention to the mark. Images, numbers, and print outlines must be clear and without deformation. If you have doubts about the authenticity of a precious metal, you can contact a specialist - a jeweler or an appraiser at a pawnshop.
Patina
Over time, a patina forms on the surface of a platinum ring, or any other piece of jewelry, regardless of the strength of the metal when interacting with the atmosphere.
In a patinated piece, the luster and color of the platinum changes slightly. What is she becoming? Even more noble. The metal develops a bluish-gray tint and a muted shine.
It has nothing to do with corrosion; it is the result of the electrochemical interaction of the alloy metals. For many people, patina is a unique and desirable feature that adds a special charm to jewelry made from this precious metal.
If you don’t like it, then jewelers, using professional pastes, can easily remove the antiquity on the product that is unwanted for the owner, returning it to its original shine.
Tips for choosing platinum jewelry
It is always better to buy jewelry in large specialized stores that have documents and value their reputation.
Resist the urge to buy platinum on the cheap. This is a fairly expensive metal. It cannot cost slightly more than silver or as much as gold. Due to the high cost of raw materials, platinum jewelry is made small in size. They are cast, not blown.
Before purchasing, we recommend that you check with the seller not only the sample, but also the composition of the ligature. Iridium is a cheaper metal. Ruthenium and cobalt add strength to the alloy.
Wedding rings
Platinum wedding rings are an ideal choice if you can afford it. Unfortunately, like many things, the best option is also the most expensive.
Introduced in the thirties of the twentieth century by an aggressive marketing strategy, the fashion for engagement rings with an indispensable diamond is a generally recognized tradition.
Platinum has become a very popular metal for making wedding rings that are worn without removal for a long time. It's perfect for a durable everyday ring.
Wedding rings are constantly exposed to household chemicals, hot water, and mechanical stress. Over time, the metal wears out, becomes scratched, and the teeth holding the stone weaken.
Platinum does not corrode, change color or lose shape over time. Being one of the hardest metals, it practically does not wear out and does not lose its shine due to surface damage during everyday wear.
Platinum prongs are more durable and hold the stone much better than gold. The risk of losing a diamond from a ring is minimal. The color and luxurious appearance of a platinum setting perfectly complements diamonds with its brilliance and brilliance.