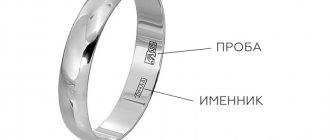
When does it become necessary to check gold for authenticity?
There are many situations when you have to determine at home whether you see real gold or counterfeit metal before your eyes. Fake gold often looks like natural metal and is an alloy of several elements. For these purposes, brass, aluminum, and iron are used. Alloys used: vermeil, pinchback, butt bronze. Sometimes, it's just well-polished copper. Another option when we can say that a precious metal is not genuine is the use of gold of a lower standard than the declared one.
Along with professional testing methods using special chemical reagents and touchstones, as well as modern analyzer instruments, there are many popular options.
Craftsmen are coming up with more and more new methods of testing noble material for authenticity. There are known identification options using vinegar, a magnet, or a lapis pencil. One of the most reliable, reliable ways: test gold with iodine at home. Explanation for this: iodine is a rare chemical element that can react with such an inert element as gold.
We purchase and use chemical reagents, standard samples and other supplies for the laboratory
Content
Acquisition and incoming control Accounting and identification Storage of reagents and standard samples
The laboratory must have the resources necessary to manage and carry out laboratory activities. Such resources, in particular chemical reagents, reference materials and other supplies purchased to support testing, require appropriate control, accounting and storage in order to prevent their use from having a negative impact on the quality of testing.
Acquisition and incoming control
The acquisition of chemical reagents, standard samples and other supplies (hereinafter referred to as materials) for the purpose of product testing is carried out by laboratories upon request
(the application form is established by the laboratory).
The purchased materials must comply with the requirements of the relevant regulatory documents and have the necessary accompanying documents (manufacturer’s passport, certificate of conformity, hygienic certificate and other documents).
Laboratory staff performs incoming inspection
accompanying documentation and materials. After analyzing the accompanying documents and their technical content, a decision is made to approve the materials for use.
Further laboratory staff:
- checks materials received against the invoice or purchase specification;
- checks the integrity of the packaging and the presence of the necessary documentation (passport, certificate, quality certificate, instructions);
- registers the received reagent in the chemical reagent log (the form of the log is determined by the laboratory);
- determines the storage location.
Return to content
Accounting and identification
The following data is reflected in the chemical reagents logbook:
- name of the reagent;
- regulatory document according to which the reagent is manufactured;
- date of arrival of the reagent;
- quantity;
- date of manufacture;
- best before date;
- signature of the employee who took the reagent, with a transcript (if the log is kept electronically, then the employee’s signature is not required).
Each container with an incoming chemical reagent is identified and assigned a number according to the list of chemical reagents.
If the container with the received chemical reagent does not have a manufacturer’s or supplier’s label, or the chemical reagent is repackaged into another container, the container must be provided with a label containing the following information:
- name of the reagent;
- provider;
- degree of purity;
- date of manufacture;
- substance content;
- chemical formula;
- best before date;
- batch number;
- Net weight;
- regulatory documentation for the chemical reagent.
Return to content
Storage of reagents and reference materials
Reagents must be stored under the conditions prescribed by the regulatory documents according to which the reagent is manufactured, and in accordance with safety guidelines for working in analytical laboratories.
Chemical reagents used in the daily work of the laboratory are stored in special cabinets in the laboratory premises.
Storage of chemical reagents in cabinets is carried out according to the list of chemical reagents, which is fixed on the inside of the cabinet doors. Laboratory personnel should be responsible for keeping this list up to date.
The consumption of reagents used in the laboratory is recorded in a log (the form of the log is determined by the laboratory).
Storage of prepared solutions and standard solutions in cabinets is also carried out according to the list of prepared solutions, which is fixed on the inside of the cabinet doors. Laboratory personnel are responsible for timely updating of the list of prepared solutions and standard solutions stored in the laboratory.
Containers with solutions are identified
assigned a number according to the list of chemical reagents and supplied with labels that contain the following information:
- name of the chemical;
- solution concentration;
- regulatory documentation for the method;
- date of preparation;
- expiration date (if such information is available in the regulatory documentation for the method).
When storing solutions of chemical reagents and standard solutions in the refrigerator, there should be a verified thermometer there. The thermometer readings are recorded daily in the temperature log
in the refrigerator (the form of the journal is determined by the laboratory).
A list of standard solutions and solutions of chemical reagents stored in the refrigerator is posted on the refrigerator.
Standard solutions and chemical solutions stored in the refrigerator are identified and labeled.
Return to content
Source link
Blog “Laboratory_” on Yandex.Zen, article “ We purchase and use chemical reagents, standard samples and other supplies for the laboratory
«.
On the “Materials” service in the “Open Development” section you can see an example of a procedure for inventory management
, in the “Document Forms” section - with ready-made templates
for record forms
for monitoring and accounting of reagents.
Necessary tools for iodine testing
A simple technology is used using tools that can be found in any home. To check you will need:
- alcohol solution of iodine, it is sold in different concentrations. The most common option is a 5% iodine solution.
- cotton swab;
- sandpaper;
- high concentration ethyl alcohol;
- a soft cloth such as flannel.
A magnifying glass and tweezers will come in handy if you need to check small details.
Iodine is able to react with gold under normal conditions, causing it to darken at the site of application. Gold iodide is formed - AuI. The dark area will have a different shade. This depends on what standard gold is: 375, 585 or 750. The latter, when combined with iodine, gives a dark green zone, while gold material of 585 standard produces a dark brown tint.
Lack of reaction is a sure sign of a fake.
Method for extracting gold from iodide solutions
(5 STATE COMMITTEE FOR INVENTIONS AND DISCOVERIES IMPRI SCST USSR DESCRIPTION OF THE INVENTION AUTHOR'S CERTIFICATE ABOUT S: nn refers to hydrometal-, losses of gold and iodine are according to be used properly 0.2-0.8 and 0.6%; ota and regeneration, ".: dldiodepartment ness process 2.5 - bch; is the formation of waste (alkaline or chemically weakly acidic solutions containing salts of one etching: potassium, i.e., the method allows regenerative regeneration to form the main component of the etching insoluble anhydrous solution, and not the solution itself), voltage 4 -5 V and There is also a known method for extracting gold (0.001 - 0.0020 b) in the form of a complex iodine-iodine composition, by adding a solution to sulfuric acid F. Khulyakov and other acids in the ratio (4-10);
They are kept at this temperature for 2 hours until the following are characteristic: loss of iodide complex. As a result of the reaction, gold is released, and the presence of oxygen, iodine sublimes and settles on the walls of the holon anode, dilation occurs. Then the mixture is cooled until temperature is reached (these are environmental requirements, metal special gold is separated from the solution. Iodine is removed from the walls of the refrigerator, degree of recovery. (54) METHOD FOR EXTRACTING GOLD IN ISODIODIDE SOLUTIONS (57) The invention relates to hydrometallurgy for the regeneration of iodide etchants. .Known for the electrical extraction of gold from a yoke of solution from the same temperature. The process is carried out at pH 4 - 12.8, temperature 50 - 600 Process 2.5-6 hours. Degree 99.2-99.8%, Degree in terms of 99.4% (cm, I" Secondary metallurgy", M: Metallurgy, This method has disadvantages: due to the high aggregation of iodine, strong corrosion is formed for the sale of materials for the electrodes 2 solutions, In a solution of iodide etchant containing 280 mg of gold. enter 18n. sodium hydroxide solution to pH 12.8, then add 0.75 ml of 30% hydrogen peroxide solution to create a concentration of 1 fg/l. The mixture is stirred, heated to boiling, kept at boiling for 0.5 hours, the gold precipitate is separated by filtration. The degree of gold precipitation is 100% with an analytical accuracy of +0 .1%. The content of gold and iodine is 99.9 and 90%, respectively (see Polish patent M 115387). The method has the following disadvantages: high energy intensity of the process (heating temperature of solutions 150 C. for at least 2 hours); complexity of the method implementation (aggressiveness of elementary 12 and especially hot solutions N 2804 impose high 10requirements for the hardware design of the method): low degree of iodine regeneration 90%; destruction of the etching solution and the formation of waste, 15 The closest technical solution to the proposed one is a method for purifying “gold”, including dissolving unrefined gold in an iodide solution, removing insoluble particles from: solution and reduction of gold to “metallic ways of bringing the pH to a value of at least 12 (see. Europatent EP M 0253783); “The disadvantages of the method include the low degree of gold recovery 95-98% 25 (another 139-1800 g of gold remains in the solution) and the duration of the process” up to 80 hours. The purpose of the invention is to increase the degree of gold recovery and speed up the process. 30 This goal is achieved in a method for extracting gold from iodide solutions, including treating the solutions with alkali metal hydroxide while stirring, followed by separation 35 of the gold precipitate, in that, unlike the typical type, alkali metal hydroxide is introduced until pH 12.8 is reached and the treatment is carried out in the presence of hydrogen peroxide in an amount of 1- 20 g/dm at a temperature of not 40 or less than 90 °C. It is known that an alkaline solution of potassium oxide reduces gold. The method is used for the qualitative determination of gold. The reduction of gold occurs according to the equation KAo 4+ ZKON = Au + 1/2 KO3 + 7/2 K + 3/2 H 20L. The reducing agent is the iodide ion, which is oxidized to iodate. According to 50 data from the prototype and the experimental results obtained, this deposition of gold is non-quantitative (92–98%), This may be due to the reversibility of reaction (1) or the formation of unknown gold compounds. It has been experimentally established that hydrogen peroxide in an amount of 1-20 g/dm at pH 12.8 accelerates the process of gold separation, allows quantitative precipitation of gold from iodide etchant, in this case, iodine losses are practically eliminated. In the proposed method, hydrogen peroxide plays the role of a catalyst. accelerating the process (1) of gold reduction with iodidione. The degree of gold precipitation does not depend on the initial concentration of hydrogen peroxide (the ratio of H202:AO is not affected). With an increase in the amount of hydrogen peroxide, when its reducing properties appear (25-60 g/l), the degree of gold precipitation decreases , finely dispersed sediment, At pH 12.8, gold precipitation is incomplete, It has been established that, in addition to pH and consumption of hydrogen peroxide, the degree of gold precipitation depends on the time and temperature at which the method is carried out. At room temperature, gold precipitation requires 8-40 hours. At a temperature of 90 C, complete precipitation gold occurs in 1 hour, including heating and cooling time. The influence of the listed parameters on the precipitation of gold from an iodide solution is illustrated in the table. As a result of the implementation of the proposed method, after the precipitation of gold, the solution contains iodide, iodate and alkali metal hydroxide. When a mineral acid is added to such a solution (after neutralization of the alkali), the following interaction of components occurs 5 + Oz + 6.H «f + ZN 20 (2) This essentially leads to the regeneration of the etching solution. The method is carried out as follows: A solution of alkaline hydroxide is introduced into the 1st etching solution with stirring metal until a pH of 12.8 is created, and then a solution of hydrogen peroxide until its concentration is 1-20 g/dm3. Heat to 90 °C (or to boiling), Maintain at this temperature for 30 minutes, settle for 30 minutes and filter, The precipitate is dried, The concentration of gold is determined in the filtrate and precipitate, The degree of gold precipitation is calculated. In the examples, an iodide etchant was used. containing, g/dm; iodine 20; h, potassium iodide 40; sodium chloride b; gold1.12.Example 1. 250 ml of iodide etchant containing 280 mg of gold were placed in a 350 ml glass. On the pH meter, bring the pH to 18 n, with a solution of eOH to 12.8 and then add 0.72 ml of a 30% solution of hydrogen peroxide (to create a concentration of H 202 - 1 g/dm3), mix and heat to boiling point, Boil for 0.5 hours, stood for 0.5 hours 30.3010.0 5.0 5.0 5.0 5.0 5.0C 30 30 30 30 30 30 Boiling. 30 90 90 22 30 30 95.0 92.0 98.0 303080 h The gold precipitate was filtered off. No gold was found in the filtrate. 280 mg of gold was found in the sediment. The degree of gold precipitation was 100%. Concentrated hydrochloric acid was added to the remaining part of the filtrate with stirring until a pH of 6.8 was created. The total iodine concentration in the filtrate was determined to be 49.6 g/dm, the degree of iodine regeneration was 98%, and the etching rate of the gold plate was determined. In the original etchant the etching rate is 11.2 mg/cm h, in the regenerated etchant the etching rate is 11 mg/cm h. The remaining examples are carried out similarly. Information about the conditions for their implementation and the achieved effect is given in the table. Examples 1.3-5, 8, 10, 13. 14 illustrate the implementation of the method using all irienacs. indicated in the claims in the claimed range of HgO2 flow rate, pH and temperature, Example 1 2, 6, 9, 15, 16, 17 illustrate the results of the experiment when implementing the method with modes; different from the claimed interval, Examples M 10-12 show the effect of temperature, Examples M 15-17 show gold deposition in accordance with the prototype. Due to the absence of the influence of pH and the consumption of gold precipitation in the prototype, more specific than pH 12 process parameters (time, temperature) experiments were carried out under conditions close to the claimed method. Thus, the proposed method for extracting gold from iodide solutions compared to the prototype has the following advantages : the degree of gold recovery increases from 95-98 to 99.9-100%; the residual concentration of gold in the solution does not exceed 1 mg/l; the duration of the process is reduced by 2-10 times; as a result of the implementation of the Method, waste is not generated, conditions are created for the regeneration of the etching solution, formula and invention Method for extracting gold from iodide solutions, including treating the solutions with alkali metal hydroxide with stirring, followed by separation of the sediment gold, characterized by the fact that, in order to increase the degree of gold extraction and speed up the process, alkali metal hydroxide is introduced until a pH of 12.8 is reached and the treatment is carried out in the presence of hydrogen peroxide in an amount of 1-20 g /dm at a temperature of at least 90 C. hydrogen peroxide to the degree of iodide etchant-ogszEu1 Notemin Part of the precipitate is finely dispersed. The sediment is finely dispersed. nyi View
The procedure for verifying the authenticity of gold with iodine. Step-by-step instruction. Video and photo
Description of the method of how to test gold at home for authenticity using iodine. Testing is easy and safe. The whole procedure takes place in several stages.
Prepare medical iodine and cotton swabs.
Selecting gold jewelry to check
Apply a drop of iodine to the decoration with a cotton swab
Wait a while until it dries
Wipe the stain with a cloth
Treat the product 585 or 583 samples with medical alcohol
At the first stage of preparation, the product is treated with medical alcohol. After processing, allow to dry.
It is recommended to first clean the jewelry with alcohol.
Sand the metal on the inside
The second preparatory stage is carried out using sandpaper. A decoration or simply an object made of gold is sanded on the side that is not the “front”. This is how the ring is processed from the inside. It is necessary to remove the top layer of metal, since it may be covered with a film of external substances. It is necessary to open the gold layer.
In addition to sandpaper, you can use any abrasive material, just not very coarse, so as not to remove too thick a layer of metal and damage the product.
Apply iodine to the jewelry in a wiped area
At the next stage, iodine is applied with a cotton swab. You can use a pipette; with it you can drop a drop exactly onto the area that has been cleaned. It is worth trying to minimize the area on which iodine falls. There will be a reaction on it, a color change will occur.
The reaction does not depend on the size of the spot, so you should not strive to get more reagent onto the surface.
Apply iodine to the cleaned surface of the jewelry
We get the result
Further stages are characterized by observation of the process. A chemical reaction occurs with a substance from the group of halogens, which includes iodine. You can test gold with iodine by its color change. Variations in brown or dark green shades indicate not only that gold is involved in the reaction, iodine indicates a sample.
Greenish tones characterize lower grade metal with a higher copper content.
How can you dissolve gold?
For many years, chemists used only a dangerous method in which, at extremely high temperatures, gold dissolves in a reaction with fluorine. But in the modern world, new, safer methods are being used.
Amalgam
Amalgam is an alloy of mercury in a liquid or solid state and is used as an industrial refining method. The process of gold amalgamation involves the ability of mercury to form compounds of several metals.
Before amalgamation, the nugget should be placed in a solution of nitric acid in a ratio of 10:1 with water. The gold must remain in solution until the visible reaction is complete, after which it must be washed.
For amalgamation, precious metal and mercury are taken in equal proportions. Place both substances in a non-metallic tray and rotate it. A ball of mercury dissolves in the molecules of the nugget. Unnecessary sediment is poured out of the tray.
The amalgam saturated with gold must be washed carefully under running water.
Excess mercury from the amalgam is removed by pressing the ball through wet suede. The compound remaining on the surface is heated until the mercury completely evaporates.
Aqua regia
Most acids have a terrible effect on organic matter. But even in them gold does not dissolve. Lomonosov's famous invention - aqua regia - is the only acid capable of starting the reaction.
Aqua regia is a mixture of hydrochloric and nitric acid in a special ratio (1:3). Its properties are greatly enhanced due to the high concentration of components.
Expert opinion
Vsevolod Kozlovsky
6 years in jewelry making. Knows everything about samples and can identify a fake in 12 seconds
The precious metal dissolves in aqua regia due to the fact that nitric acid oxidizes hydrochloric acid. A special compound is formed - atomic chlorine, which instantly reacts with the metal, creating complex chlorauric acid. Part of the precious metal crystallizes, and the other part dissolves.
It is worth noting that the occurrence of a chemical reaction depends on what acid the metal is dissolved in and what its concentration is.
Bleaching
Chlorine, widely used in everyday life, is an aqueous solution of chlorine gas, which belongs to the group of halogens. For refining, bleach purchased in a regular store is not suitable, because... its concentration is too low.
A concentrated solution of chlorine has the following effect: chlorine breaks down into hydrochloric and hypochlorous acids, the second, in turn, under the influence of sunlight, decomposes into hydrochloric acid and oxygen. As in the reaction with aqua regia, an atomic substance is released that easily oxidizes the nugget.
Iodine itself is an insoluble substance in water. Its compound with potassium iodide dissolves. This is a medicine called Lugol.
Gold dissolves in Lugol due to the fact that iodine creates weak compounds - anions. But the reaction is much slower than with acids, and only the top layer of metal dissolves.
Which method is suitable for home use?
Gold refining (obtaining pure metal) can be done at home. The safest method of dissolution is using electric current.
Electrolysis
A large bath is filled with hydrochloric acid and gold chloride, a reagent used to test products and determine the sample. It can be isolated using aqua regia, gold and ammonia or purchased ready-made in jewelry supply stores.
The reaction, called electrochemical, occurs due to the voltage applied to the bath. As a result, high-grade metal without impurities settles on the sides, and the remaining components precipitate at the bottom of the bath.
Other verification tools
Among other means of checking the authenticity of precious metals, there are also several simple options.
Magnet
Checking with a magnet. The property of gold being diamagnetic is used. This is a substance that does not react to a magnetic field. Moreover, pure metal will repel the magnet. Other metals, with the exception of precious metals, have to one degree or another the properties of ferromagnets that experience mutual attraction with a magnet.
Therefore, gold of low purity with additives of other metals can react to a magnet. If the product “sticks” to it, this is a sign of the use of a ferromagnetic alloy with iron, nickel, and other metals.
In this way, you can identify counterfeit jewelry or detect a lower standard at home.
There is the possibility of counterfeits made from materials such as copper and aluminum since they are not magnetic. However, in the presence of a strong magnet, a field arises between it and the copper, which is triggered in such a way that when approaching the metal and almost touching it, the magnet stops abruptly.
Gold in its pure form is not attracted to a magnet
Lapis pencil
A drug based on silver and potassium nitrates is useful for testing gold. To do this, the decoration is lowered under water or part of it is wetted, and a strip is applied to the wet area with a pencil. Then wipe with a dry cloth or cotton pad. The trace left from the lapis indicates that the product is counterfeit.
There will be no stripes left on the gold.
The most common type of lapis pencil
How to dissolve gold with chlorine at home
The use of chlorine in the ore hydrometallurgical cycle of gold production has a fairly long history. From the middle to the end (80-90s) of the 19th century, chlorination leaching of gold, based on the formation of a water-soluble chlorine complex AuCl-4 according to the reaction: 2Au+3Cl2+2Cl-=2AuCl-4,
was the main and practically the only method of hydrometallurgical processing of gold ores.
The first experimental work on the extraction of gold with chlorine was carried out by Percy, who reported on them in 1848. The first factory chlorination installation was created in 1849 in Reichestein. In subsequent years, a number of plants were built in the USA and Australia for the leaching of gold from ores with chlorine using various options for the instrumental design of the process /1/.
Chlorination with chlorine gas was usually carried out under low pressure in a series of wooden percolation tanks arranged in series with tight leaded lids and false bottoms, on the surface of which a layer of gravel and sand was poured. Chlorine was fed into the lower part of the vat (under the sand filter) and the ore was soaked in it for 12-36 hours. After this, the vat was filled with water. The excess chlorine displaced in this case entered the subsequent chlorination tank through the lead outlet pipe in the upper part of the vat. After saturating the loading of the last vat with chlorine, its excess was forced into the 1st vat, and this completed the chlorination cycle. This system ensured the maximum degree of use of chlorine, the consumption of which when processing pre-calcined (to remove sulfide sulfur) ore was less than 1% of the mass of the processed material.
An improved version of chlorination involved the use of metal rotating drums (“barrels”) made of iron and lined with lead on the inside as the main reaction apparatus. The devices had a capacity of 12 to 18 tons of ore and operated in batch mode. The advantages of this method of chlorination, compared to the previous one, were:
— grinding of ore material during leaching (which is of particular importance for removing silver chloride films from the surface of gold particles);
— ability to work at higher chlorine pressure;
— the use of other chlorine-containing reagents instead of gaseous chlorine, in particular, bleach, which generates Cl2 as a result of the interaction of Ca(OCl)2 with sulfuric acid introduced into the process.
The chlorination process in barrels was carried out as follows. First, water was poured into the barrel, then bleach was introduced and roasted ore was loaded on top. After this, sulfuric acid was poured onto the ore layer, and the lid covering the hatch was tightly bolted.
According to the experience of the Cripple Creek gold recovery plant (USA) and some other facilities practicing this technology, the consumption of chlorine in the process of “barrel” chlorination of roasted gold and silver ores was 0.15-0.5% of the mass of the original ore.
The most significant scale of chlorination leaching of gold was achieved at the Mount Morgan factory (Australia), where this process was carried out in open percolation tanks by leaching ore with chlorine water. The ore was dry ground in ball mills to minus 20 mesh (0.84 mm) and fired in rotary cylindrical kilns. The roasted ore was leached in open rectangular concrete vats with a capacity of 100 tons. The chlorine water required for the process was produced from chlorine gas by passing it through scrubbers, followed by the accumulation of chlorine water in closed containers, from where it was then supplied to irrigate the ore into open percolator vats. The solution (with a chlorine concentration of 1.4 g/l) was poured into the vats in such a quantity that its level was higher than the ore level. The contact of the ore with the solution continued for 36–64 hours, with a chlorine consumption of 1.3 kg per 1 ton of ore. Gold precipitation from solutions was carried out by adsorption on charcoal “filters” according to the reaction:
4AuCl3 + 3C + 6H2O = 4Au + 12HCl + 3CO2
The recovery of gold by the described process, depending on the nature of the ore, ranged from 92 to 95%,
In addition to carbon adsorption, as has been established by numerous experimental studies, the extraction of gold from chloride solutions can also be carried out by chemical methods using iron (I) sulfate, sulfur dioxide, as well as hydrogen sulfide and heavy metal sulfides (CuS, PbS, FeS) as metal precipitants. . In the first two options (FeSO4, SO2) gold is deposited in the form of a metal, in other cases - in the form of Au2S3 sulfide.
The above description of the previously used hydrochloric acid technology for extracting gold from ores naturally corresponded to the technical level of gold production of that period. However, many aspects of this technology have not lost their relevance today due to the newly revived interest in the use of chlorine in gold hydrometallurgy (which will be discussed in subsequent sections of the article).
The cardinal factor that determined the fate of the hydrochlorination method of gold extraction was the creation (1887-1889) and subsequent rapid development of the cyanide leaching process. It can be considered that with the development of cyanidation technology, the largest technical revolution in the global gold mining industry took place. Thanks to its technological, economic, and also, strange as it may seem at first glance, environmental advantages (see the Bulletin "Gold Mining", issues 112-114, 2008), the cyanide process has firmly taken a leading place in the industrial practice of gold production from ores of primary deposits, displacing all other alternative hydrometallurgical options, including chlorination.
By 1918, there was not a single operating installation left in the world for the chlorination leaching of gold from ores or ore concentrates.
At the same time, intensive research continued in the USSR and abroad to study the theoretical and technological aspects of the hydrochlorination process and compare the indicators of this process with cyanidation of gold ores and concentrates. At the same time, a number of advantages of hydrochlorination were established, which made it possible to assess with a sufficient degree of optimism the prospects for its use in the gold mining industry. These include /2/:
a) a higher rate of leaching of gold with chlorine chloride solutions due to the use of high concentrations of the oxidizing agent (molecular chlorine). For example, I.A. Kakovsky (1975) determined that under comparable conditions the specific rate of gold dissolution during chlorination is 13 times higher than during cyanidation using oxygen, and 43 (!) times higher than during cyanidation with blowing air.
b) the possibility of obtaining hydrochloric acid solutions rich in Au content, from which it is subsequently convenient to extract gold by direct electrolysis.
c) the effectiveness of applying chlorination leaching of gold to various ore materials that are difficult to cyanide, for example, antimony, arsenic, cuprous, telluric concentrates.
However, as relevant technological developments have shown, the advantages of hydrochlorination listed above, however, cannot fully compensate for such main disadvantages of this process as the need to use aggressive chemical environments (respectively, corrosion-resistant structural materials) and, especially, the high consumption of chlorine in hydrometallurgical cycle. The latter circumstance is due to the fact that, unlike alkaline cyanide solutions (usually with a very low solvent concentration - NaCN), acidic chlorine-containing solutions have a pronounced collective dissolving effect in relation to a large group of mineral components, and, above all, sulfides.
This fact is reflected in the above examples of the industrial use of chlorination, when pre-roasted ores with a minimum content of sulfide sulfur were subjected to treatment by this method.
In addition to sulfides, active “consumers” of chlorine are carbonates and oxides of alkali metals (CaO, MgO, etc.), iron sulfates, talc and other chemical compounds present in the feedstock or formed during the oxidative roasting of gold ores (concentrates).
Along with the high consumption of chlorine, during chlorination leaching of such materials, solutions with a very high salt background are formed, the subsequent chemical treatment of which causes much more complex problems compared to the treatment of cyanide-containing tailings and wastewater.
For the reasons stated above, chlorine cannot yet be considered as an equivalent substitute for cyanide in the hydrometallurgical production of gold (as well as silver) from ore raw materials.
At present, only isolated, very few examples of this kind are known. One of them is chlorination leaching of gold from “slag” antimony concentrate (cinder of oxidative sublimation roasting of antimony) in South Africa /3/. According to other information /2/, this process was used in the processing of ore containing gold tellurides at the Emperor Gold Mine enterprise (Fiji).
At the same time, the possibility of using chlorine in gold hydrometallurgy, not as a substitute for cyanide, but as a reagent in technological operations that complement the cyanidation process, has been discovered and has received corresponding development. These include:
— chlorine neutralization of cyanide leaching tailings;
— the use of chlorine to neutralize sorption-active carbon during cyanidation of technologically resistant carbonaceous gold ores and concentrates.
The method of detoxifying cyanide with chlorine is dominant in the domestic practice of processing gold ore raw materials and is quite widely used abroad. Compared to other alternative methods for neutralizing cyanide-containing liquid waste, the chlorination process provides the most complete purification of solutions from both cyanide (CN—) and thiocyanates (CNS—). A significant contribution to the development and mastery of chlorination technology for the neutralization of cyanide-containing waste and to the study of the processes of natural degradation of cyanides was made by specialists from the Environmental Protection laboratory established in 1967 in Irgiredmet.
The process of chemical passivation of sorption-active carbon with chlorine before cyanidation of carbonaceous gold ores was developed and implemented at several US enterprises in the 70-80s of the last century. The most interesting examples of industrial use of this option are the Jerritt Canyon and Carlin gold mining plants.
Jerritt Canyon processes two types of ore: carbonaceous (Au 8.5 g/t) and oxidized (5.8 g/t). Carbonaceous ores are characterized by a high content of organocarbon material and pyrite, with which some gold is associated. Oxidized ores contain less organic carbon and more carbonate minerals: calcite, dolomite, etc.
In 1983, the plant introduced joint processing of both types of ore. According to the accepted technology, carbonaceous ore (a condensed grinding product with a density of 53% solid) is subjected to oxidation in an atmosphere of chlorine. The process takes place for 16–20 hours at 70–80°C and slight excess pressure. To neutralize the sulfuric acid formed during the oxidation of pyrite, soda is supplied.
Chlorination is carried out in sealed containers. Unreacted chlorine is captured in a carbonate-bicarbonate solution and returned as bleach to the process. Chlorine consumption is 12–23 kg per 1 ton of ore. After this pre-treatment, the pulp is sent for cyanidation, carried out using the CIL method (sorption leaching with granular activated carbon), together with the oxidized ore. The combination of chlorine oxidation and sorption cyanidation processes ensures the recovery of gold from a mixture of ores (Au 6.7 g/t) at a level of 92%.
At the Carlin factory, in connection with the involvement of refractory carbon-containing ores into production, a separate section for their processing was launched in 1974 (540 tons per day). The ore (Au 5.5 g/t) is subjected to chlorination by purging the pulp with chlorine gas for 20 hours at 27–38°C. Chlorination tanks are sealed and operate by recycling the released Cl2. In order to reduce chlorine consumption (which initially reached 200 kg per 1 ton of ore), the plant implemented a “flare” chlorination process and, in addition, implemented “double oxidation” technology. The essence of the technology is that ore in the form of pulp (40–50% solid) is initially treated with air in 4 successively located vats, with the addition of soda (25 kg per 1 ton of solid) when heated with live steam to 80°C. The resulting pulp is cooled to 50°C, after which it is treated with chlorine sequentially in 6 vats. The chlorine consumption is approximately 25 kg per 1 ton of ore. The chlorinated pulp is supplied for cyanidation together with crushed oxidized ore. The extraction of gold in the cyanide cycle from carbon ore is 86.9%, from oxidized ore - 88%, which indicates a fairly high degree of suppression of sorption-active carbon in the process of “double oxidation”.
There are also other examples of the use of hydrochlorination, which will be discussed later.
LITERATURE
1. Plaksin I.N. Metallurgy of noble metals.-M.: Metallurgizdat, 1943.-420 p.
2. Kotlyar Yu.A., Meretukov M.A., Strizhko L.S. Metallurgy of precious metals: Textbook in 2 books.-M.: MISIS, Publishing House "Ore and Metals", 2005.-Book.2.-392 p.
3. Lodeyshchikov V.V. Technology for extracting gold and silver from refractory ores. In 2 t.-Irkutsk; Irgiredmet, 1999.-786 p.
Convenience of the method and its disadvantages
A household method of checking precious metal for authenticity using iodine:
Convenient and easy enough to use.
Does not require complex chemical reagents or special equipment.
It can be carried out by non-specialists; it is enough to carefully observe the reaction of the interaction of two elements.
The disadvantages include:
Checking only the surface part of the jewelry.
It is impossible to obtain a result with 100% accuracy.
It is impossible to check gold-plated products; the top layer of gold will be removed with sandpaper.
Difficulties also arise with rhodium-plated products, if a thin layer of rhodium is removed, a yellowish or reddish metal appears underneath it.
Jeweler services as a reliable and safe way to verify the authenticity of metal
Traditional methods of metal testing are good as an express method. For reliability and confidence in the results, you should contact a professional jeweler. In his arsenal there will be all the necessary means of determining the authenticity of a gold product.
The jeweler always has the necessary tools at hand to test precious metals.