CADMIUM
(Cadmium) Cd is a chemical element of group II of the Periodic table.
Atomic number 48, relative atomic mass 112.41. Natural cadmium consists of eight stable isotopes: 106Cd (1.22%), 108Cd (0.88%), 110Cd (12.39%), 111Cd (12.75%), 112Cd (24.07%), 113Cd ( 12.26%), 114Cd (28.85%) and 116Cd (7.58%). Oxidation state +2, rarely +1. Also on topic:
CHEMICAL ELEMENTS
Cadmium was discovered in 1817 by the German chemist Friedrich Stromeyer Friedrich (1776–1835).
When checking the zinc oxide produced by one of the Schenebec factories, it was suspected that it contained an admixture of arsenic. When the drug was dissolved in acid and hydrogen sulfide was passed through the solution, a yellow precipitate similar to arsenic sulfides formed, but a more thorough check showed that this element was not present. For the final conclusion, a sample of suspicious zinc oxide and other zinc preparations (including zinc carbonate) from the same factory were sent to Friedrich Strohmeyer, who from 1802 held the chair of chemistry at the University of Göttingen and the position of inspector general of Hanoverian pharmacies.
Also on topic:
PERIODIC SYSTEM OF ELEMENTS
Having calcined zinc carbonate, Strohmeyer obtained an oxide, but not white, as it should have been, but yellowish. He assumed that the color was caused by an admixture of iron, but it turned out that there was no iron. Strohmeyer completely analyzed zinc preparations and found that the yellow color appeared due to a new element. It was named after the zinc ore in which it was found: the Greek word kadmeia, “cadmium earth,” is the ancient name for smithsonite ZnCO3. This word, according to legend, comes from the name of the Phoenician Cadmus, who allegedly was the first to find zinc stone and notice its ability to give copper (when smelted from ore) a golden color. The same name was given to the hero of ancient Greek mythology: according to one legend, Cadmus defeated the Dragon in a difficult duel and on his lands built the fortress of Cadmea, around which the seven-gate city of Thebes then grew.
Vigilant Apothecary
The story began in Magdeburg with a district doctor-inspector. He ordered drugs containing zinc oxide to be withdrawn from sale because they allegedly contained arsenic. The pharmacists were indignant and turned to F. Strohmeyer, the chief inspector of pharmacies.
Elemental cadmium
The drugs were examined and no arsenic was found. But they discovered the oxide of a new element. Strohmeyer named the discovered element "cadmium", after the hero of the ancient Greek myth.
History of discovery
The “fathers” of the element in the history of science included German pharmacists and chemist Friedrich Strohmeyer.
In 1817, pharmacists, examining zinc oxide, saw in its composition characteristics inherent in arsenic. Strohmeyer carried out a series of reactions at their request. The end product was not arsenic, but a new silvery metal.
It was dubbed cadmium - after the name of the local zinc-containing ore.
Cadmus is a character in ancient Greek mythology, the legendary founder and king of Thebes.
Properties of Cadmium
Cadmium is a transition metal.
It is soft, viscous and malleable. Characteristics:
- The crystal lattice structure is hexagonal.
- The element has a record thermal conductivity for metals in the region of absolute zero.
- Color white, silver.
Cadmium is a heavy metal, along with osmium, lead, and molybdenum.
Has three modifications; They can be distinguished by certain physical parameters.
Chemical properties:
- Cadmium oxides and sulfides are almost insoluble in water.
- In humid air conditions, an oxide film forms on the surface. It prevents further oxidation.
- Does not form carbides.
- In heated dilute H2SO4 and HNO3 (formulas of sulfuric and hydrochloric acids), cadmium dissolves, releasing hydrogen.
| Properties of the atom | |
| Name, symbol, number | Cadmium (Cd), 48 |
| Atomic mass (molar mass) | 112.411(8)[1] a. e.m. (g/mol) |
| Electronic configuration | [Kr] 4d10 5s2 |
| Atomic radius | 154 pm |
| Chemical properties | |
| Covalent radius | 148 pm |
| Ion radius | (+2e) 97 pm |
| Electronegativity | 1.69 (Pauling scale) |
| Electrode potential | −0,403[2] |
| Oxidation states | 2 |
| Ionization energy (first electron) | 867.2 (8.99) kJ/mol (eV) |
| Thermodynamic properties of a simple substance | |
| Density (at normal conditions) | 8.65 g/cm³ |
| Melting temperature | 594.1 K (321 °C) |
| Boiling temperature | 1038 K (764.85 °C) |
| Ud. heat of fusion | 6.11 kJ/mol |
| Ud. heat of vaporization | 59.1 kJ/mol |
| Molar heat capacity | 26.0[2] J/(K mol) |
| Molar volume | 13.1 cm³/mol |
| Crystal lattice of a simple substance | |
| Lattice structure | hexagonal |
| Lattice parameters | a=2.979 c=5.618 Å |
| c/a ratio | 1,886 |
| Debye temperature | 209 K |
| Other characteristics | |
| Thermal conductivity | (300 K) 96.9 W/(mK) |
| CAS number | 7440-43-9 |
We recommend: ALUMINUM - the roads it chooses
Biological effects
Microdoses of cadmium are found in biological organisms: the substance promotes metabolism.
Toxicity
However, the metal and its soluble compounds are toxic:
- The most toxic vapors are dusty and smoky air.
- The substance accumulates in the human body, plants, and fungi.
- Heavy metal and its compounds provoke cancer and inhibit the functioning of the hormonal, circulatory, and central nervous systems. Bones are destroyed.
The effect on the body is similar to mercury and zinc, but the toxicity is two to three times higher than zinc.
Cadmium is classified as a hazard class 2 substance. 7.50 g of metal in a cubic meter of air kills in twenty seconds.
Norms
The presence of metal in water and food products is regulated by sanitary standards of the Russian Federation (µg/kg):
- beef, pork – 16;
- bread – 30;
- rice – 60;
- fish – 200;
- cocoa – 500;
- offal – 1000.
A liter of drinking water cannot contain more than 0.001 mg of cadmium.
How to produce
Production is associated with the production of zinc. Cadmium is a by-product of the extraction of zinc from its minerals. This method of production is amalgam.
The hydroelectrometallurgical method consists of the following stages:
- ore leaching;
- cleaning the solution from impurities;
- carburization or electrolytic deposition of metal;
- melting the resulting sponge.
Then, if necessary, the metal is refined and brought to high purity.
Receiving technology
- Cadmium is mined from zinc ores. The metal is recovered from by-products. Here it accumulates during ore processing.
- The second method of production is from copper-lead dust. The dust is treated with sulfuric acid, then cadmium sulfide is leached with water. Pure metal is obtained by electrolysis or reduction with zinc.
Advantages and disadvantages
| Advantages of cadmium products | Flaws |
| Performs well in environments containing Cl (for example, sea water) | It is useless to cadmium-plate products operating in the presence of sulfur and its compounds |
| Have high ductility | Welding destroys cadmium coatings |
| Allowed to be paired with oxidized aluminum and zinc | Cadmium and its compounds are hazardous to health |
Being in nature
No cadmium deposits have been found on the planet: the rare element is scattered throughout the earth’s crust, water, and air.
A ton of the earth's crust contains 130 mg of cadmium, a liter of sea water - 0.00011 mg.
The second form of metal occurrence in nature is its six minerals.
More often, cadmium accompanies other minerals (fifty). This is how it is mined - along with other ores. Particularly rich are zinc (cadmium is always present here), lead, and copper deposits.
Application
Most cadmium coatings are used to protect:
- products used in sea water;
- machine parts that operate in high humidity conditions;
- protect electrical contacts from corrosion.
Important: the use of cadmium coatings in household products is prohibited due to the high toxicity of the metal.
A lot of cadmium produced is used in low-melting alloys, anti-friction alloys, and in alloys with precious metals.
A fifth of the mined metal is used in batteries and accumulators.
The nuclear industry uses cadmium to produce control rods. Included in neutron radiation protection screens.
We recommend: GOLD - a gift or curse to humanity
The production of CdTe solar cells is on the rise.
Part of the metal is used to produce coloring pigments.
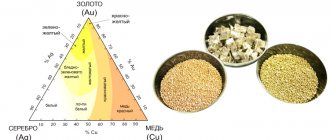
Alloys with gold are used in jewelry production. Depending on the percentage of components, they have colors from bright yellow to greenish.
Radioactive isotopes are used in the treatment of cancer.
Important: toxic cadmium may be contained in tin soldiers that children play with.
Where is it used?
Toxicity limits, but does not nullify the benefits of the metal. It is used in all segments except the food industry.
Industry
Here metal was used as an independent unit and a component of alloys:
- Anti-corrosion coatings. Cadmium plating provides the strongest, tenacious connection to the base. Used in extreme conditions: sea water, tropics, alkaline environment.
- Production of batteries, accumulators for rockets.
- Alloying additive to alloys. Alloys with cadmium are ductile and wear resistant. They are used to make wires for power lines, solders, and bearings for filling sea and airliners. The alloys are used for soldering glass, metal, and in fire extinguishers.
- Component of semiconductors, solar cell films.
Metal salts become pigments (yellow color). But this is not always welcome.
Coca-Cola has withdrawn more than 20,000 glasses with its logo from the US market. The reason is the presence of cadmium in the paint.
The most important area of application is the nuclear complex:
- Cadmium rods regulate the operation of the reactor.
- Screens that cut off neutron radiation are made of it.
In laboratories, metal is used as a reagent.
Other industries
Good thermal conductivity at extremely low temperatures has made the metal indispensable in cryotechnics.
Jewelers add cadmium to gold to create greenish tints.
The beneficial property of the substance to accumulate in cancer cells is used in antitumor therapy.
The annual global production of the metal is estimated at 20 thousand tons.
The price of a ton of cadmium on world markets is $2.8-2.9 thousand.
Restrictions
According to the Technical Regulations of the Eurasian Economic Union and the European Union Directive, products in the electrical and radio electronics segment should contain no more than 0.01% of the mass fraction of cadmium.
[edit] Literature
- Glossary of terms in chemistry // J. Opeida, O. Shvaika. Institute of Physical-Organic Chemistry and Coal Chemistry named after. L. M. Litvinenko NAS of Ukraine, Donetsk National University - Donetsk: "Weber", 2008. - 758 p. ISBN 978-966-335-206-0
- Small mountain encyclopedia. In 3 volumes / Ed. V. S. Beletsky. - Donetsk: Donbass, 2004. - ISBN 966-7804-14-3
- R. Ripan, I. Certeanu. Inorganic chemistry: Chemistry of metals: In 2 vols. - M.: Publishing house. "Mir", 1971. - T. 1. - 561 p.
- Chemical properties of inorganic substances: Textbook. manual for universities. 3rd ed., Rev. / R. A. Lidin, V. A. Molochko, L. L. Andreeva; Ed. R. A. Lidin. - M.: Chemistry, 2000. 480 p.: ill. — ISBN 5-7245-1163-0.
Electrochemical activity series of metals
Eu, Sm, Li, Cs, Rb, K, Ra, Ba, Sr, Ca, Na, Ac, La, Ce, Pr, Nd, Pm, Gd, Tb, Mg, Y, Dy, Am, Ho, Er, Tm, Lu, Sc, Pu, Th, Np, U, Hf, Be, Al, Ti, Zr, Yb, Mn, V, Nb, Pa, Cr, Zn, Ga, Fe, Cd, In, Tl, Co, Ni, Te, Mo, Sn, Pb, H2
,W, Sb, Bi, Ge, Re, Cu, Tc, Te, Rh, Po, Hg, Ag, Pd, Os, Ir, Pt, Au
| Periodic table of chemical elements by D. I. Mendeleev | |||||||||||||||||||||||||||||||
| H | He | ||||||||||||||||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||
| Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | MD | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| Uue | Ubn | Ubu | Ubb | Ubt | Ubq | UBP | Ubh | ||||||||||||||||||||||||
| Alkali metals | Alkaline earth metals | Lanthanides | Actinoids | Superactinoids | Transition metals | Other metals | Semimetals | Other non-metals | Halogens | Noble gases | Properties unknown |
How is it dangerous for humans?
Thermal conductivity of copper
People are poisoned by cadmium by consuming water and grains and vegetables growing on lands located near oil refineries and metallurgical plants. Unbearable muscle pain, involuntary bone fractures (cadmium can wash calcium out of the body), skeletal deformation, dysfunction of the lungs, kidneys and other organs appear. Excess cadmium can cause malignant tumors.
The carcinogenic effect of nicotine in tobacco smoke is usually associated with the presence of cadmium.
Cadmium is excreted in feces and urine, but not more than 48 mg per day. Most of all it accumulates in the liver and kidneys, and a little in the blood.
The more developed industry is in a country, the greater, unfortunately, is the concentration of this element in the soil. In the presence of superphosphates, plants absorb cadmium in large quantities, and if there are few superphosphates, then cadmium may not be absorbed or absorbed in minimal quantities.
Links Edit
Periodic table of elements
| H | He | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Cs | Ba | * | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | ** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Uub | Uut | Uuq | Uup | Uuh | Uus | Uuo |
| * | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||
| ** | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | MD | No | Lr |
| What is friction force in physics Select Cadmium and find in: |
|
- Page 0 - short article
- Page - encyclopedic article
- Miscellaneous - on pages: , , ,
Isotopes
Natural cadmium consists of 6 stable isotopes. Twenty-seven stable radioisotopes were identified: Cd-113 with a half-life of 7.7 quadrillion years, Cd-109 with a half-life of 462.6 days, and Cd-115 with a half-life of 53.46 hours. All other radioactive isotopes have a half-life of less than 2.5 hours and most of them have a half-life of less than 5 minutes. This element has 8 metastable states, the most stable of which are: Cd-113 (t ½ 14.1 years), Cd-115 (t ½ 44.6 days) and Cd-117 (t ½ 3.36 hours).
Cadmium isotopes have atomic masses ranging from 96.935 Dn (Cd-97) to 129.934 Dn (Cd-138). The main mode of decay of the most common stable isotope Cd-112 is the capture of an electron and its beta radiation. The decay product before the operation is element 47 (silver), and after - element 49 (indium).
general information
The simple substance is cadmium. Soft malleable metal, allotropic modifications NOT ma... Gustina 8.65, melting point 321.1 ° C, boiling point - 766.5 ° C. Clark K. - 1.35 · 10-35%. Reacts with acids. Soluble compounds are poisonous. Forms rare minerals: greenockite CdS (77.7% Cd), otavite CdCO 3, cadmoselite CdSe, monteponite CdO (87.5% Cd). It occurs as an isomorphic impurity in zinc minerals, especially sphalerite. An admixture of Cd (thousandths percent) is found in hydrothermal ores, where it is present in sphalerite, galena, etc., mainly sulfide minerals. An increased K content of up to 1.5% is typical for low-iron sphalerite.
In humid air it becomes covered with a protective oxidation film with CdO, and upon strong heating it burns to CdO. It is easily oxidized to halide by halogens. Dissolves in mineral acids, insoluble in meadows. Reacts with oxygen when heated, as well as with acids.
Notes
- ↑ Chemical Encyclopedia: in 5 volumes / Editorial Board: Knunyants I. L. (chief editor). - M.: Soviet Encyclopedia, 1990. - T. 2. - P. 280. - 671 p. — 100,000 copies.
- . — Description of cadmium compounds, its discovery, etc. Retrieved May 16, 2010.
- Cadmium // Kazakhstan. National Encyclopedia. - Almaty: Kazakh encyclopedias, 2005. - T. III. — ISBN 9965-9746-4-0.
- VVER-1000: physical principles of operation, nuclear fuel, safety / A.M. Afrov, S.A. Andrushechko, V.F. Ukraintsev et al. - M.: University Book, Logos, 2006. - P. 45.
- . Decision of the Council of the Eurasian Economic Commission of October 18, 2016 N 113. Date of access: April 19, 2022.
- . — Cadmium on the Chemist's Handbook website. Retrieved May 16, 2010.
- ↑
- . Interfax (June 4, 2010). Retrieved June 4, 2010.