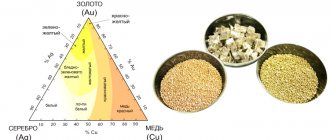
In a previous article, TD Serebro talked about why some metals are considered precious. It turned out that there is a simple explanation for this: they have unique chemical, physical and aesthetic properties.
Each metal is unique in its own way, but our company treats noble silver with special respect. It became the basis of the trading house’s assortment, is one of the main metals in jewelry and is very widely in demand in light and heavy industry. There are amazing facts and several myths associated with silver - we will confirm some, dispel others, and all of this will be interesting.
Disclaimer: We do not try to tell everything, but want to give you a general understanding of those things that will be useful when choosing jewelry. We are a jewelry company. If you want to understand these issues in depth, you might want to consider special education.
Physics
Silver is a white precious metal. Its density is 10.5 g/cm³, temperature 960.5°C, boiling point 2210°C, Brinell hardness in the annealed state (after annealing) 25 kgf/mm². These numbers mean little to a non-specialist, but they are very important for those involved in processing the material. Depending on these indicators, different forces and methods are applied to the metal to give the finished products the desired properties.
Annealing is a high-temperature treatment to give the metal a more stable state, eliminate inhomogeneities, and relieve stress due to deformation. Silver alloys are annealed at a temperature of 600–650°C for up to ten minutes, depending on the mass, and with rapid cooling. Almost all silver jewelry is annealed.
Silver polishes well, is highly reflective, has good malleability and the highest thermal and electrical conductivity of all metals.
APPENDIX A (for reference). Properties of gold-based alloys
APPENDIX A (for reference)
Table A.1
| Brand | Estimated density, g/cm | Melting point (range), °C | Hardness НV*, kgf/mm | Color | |
| Solid | Soft | ||||
| ZlSrM 375−20 | 11,24 | 965−985 | 235 | 130 | Bright yellow |
| ZlSrM 375−100 | 11,41 | 925−940 | 265 | 155 | Red |
| ZlSrM 375−160 | 11,54 | 880−900 | 240 | 150 | Red |
| ZlSrM 375−250 | 11,74 | 770−850 | 250 | 150 | Pink-yellow |
| ZlSrPdM 375−100−38 | 11,56 | 850−975 | 230 | 155 | Yellowish orange |
| ZlSrM 500−100 | 12,47 | 835−925 | 240 | 160 | Red |
| ZlSr 585−415 | 14,30 | 1025−1030 | 115 | 40 | Green |
| ZlSrM 585−80 | 13,24 | 880−905 | 270 | 170 | Red |
| ZlSrM 585−200 | 13,60 | 830−845 | 270 | 170 | Reddish yellow |
| ZlSrM 585−300 | 13,92 | 835−880 | 250 | 150 | Yellow-green |
| ZlSrPd 585−255−160 | 14,76 | 1175−1220 | 185 | 75 | White |
| ZlSrPdTs 585−287−100 | 14,31 | 1150−1180 | 160 | 70 | White |
| ZlSrPdKd 585−280−100 | 14,60 | 1160−1190 | 140 | 110 | White |
| ZlSrNTsM 585−80−8.2−2.5 | 13,11 | 825−1015 | 330 | 230 | Light yellow |
| ZlNTsM 585−12.5−4 | 12,85 | 870−950 | 300 | 170 | White |
| ZlSr 750−250 | 15,96 | 1040−1045 | 115 | 40 | Green |
| ZlSrM 750−125 | 15,45 | 885−900 | 270 | 140 | Bright yellow |
| ZlSrM 750−150 | 15,53 | 890−920 | 250 | 130 | Yellow |
| ZlSrNC 750−150−7.5 | 15,38 | 900−950 | 240 | 140 | White |
| ZlSrPd 750−100−150 | 16,44 | 1250−1300 | 150 | 85 | White |
| ZlSrPlM 750−80−90 | 16,78 | 955−1055 | 180 | 125 | Yellowish |
| ZlSrPdN 750−90−140 | 16,32 | 1155−1247 | 180 | 140 | White |
| ZlSrPdN 750−70−140 | 16,25 | 1115−1215 | 195 | 150 | White |
| ZlSrPdNKd 750−90−85−4 | 15,80 | 1140−1170 | 190 | 135 | White |
| ZlNTsM 750−7.5−2.5 | 14,81 | 910−950 | 200 | 150 | White |
| ZlSrM 958−20 | 18,52 | 1005−1030 | 140 | 50 | Bright yellow |
| ZL 999 | 19,30 | 1063 | 50 | 30 | Bright yellow |
| PLN 999.9 | 19,30 | 1063 | 50 | 30 | Bright yellow |
| _______________ *Hardness was determined on samples with a degree of deformation of 75−95%. | |||||
Table A.1. (Changed edition, Amendment No. 2).
Chemistry
Silver is stable in water, practically does not react with oxygen in the air at room temperature, but due to the presence of hydrogen sulfide in the air, over time it becomes covered with a thin dark coating of silver sulfide. Silver also reacts with ozone, forming a coating of silver oxide. This is the very darkening that is considered the main drawback of this metal.
Copper, which is the most common alloy for silver alloys, also forms a coating of copper sulfide. The higher the copper content in the alloy, the faster the product will darken, and the lower, the less susceptible the alloy is to tarnishing, so alloys from 875 to 960 are considered the most stable.
Ligature is an alloy of two or more components that is added to a precious metal to bring the jewelry alloy to a certain standard, change the color of the alloy, and also impart various useful properties. The process of adding a ligature is called doping.
Silver dissolves in nitric and hot concentrated sulfuric acid. Like gold, it reacts with alkaline solutions of cyanide. You will probably never encounter these things. And if you do, be very careful.
How to determine authenticity
If you have doubts about the authenticity of the product, you can check it at home:
Pass the decoration along a sheet of paper. Silver jewelry will leave streaks.- Draw a thin line of iodine with a needle. If iodine leaves yellow spots, the product is real. A white coating indicates a fake.
- Silver heats up quickly. If you immerse the product in hot water for a few seconds and then remove it with tweezers, it will be the same temperature as the water in the glass.
- Genuine jewelry is not magnetic.
- You can run chalk over the surface of the product. If the chalk becomes dark, the decoration is genuine.
There are no fakes in official stores. When purchasing, you need to pay attention to the reputation of the store. The low price of the product should also alert the buyer.
Silver alloys
Pure silver is a heavy (lighter than lead, but heavier than copper and harder than gold), unusually ductile silvery-white metal with a light reflectance of about 100%, therefore, in its pure form, silver is usually used only to cover jewelry made from silver alloys, base metals, etc. component of gold and silver alloys and solders.
In the manufacture of jewelry, in order to increase the hardness and strength of the material, silver is processed in alloys with other metals. Most often these are two-component alloys of silver and copper in varying percentages and with a small amount of impurities.
Silver alloys vary slightly in shade and have approximately the same mechanical properties. Tableware items and decorative table decorations are made from alloys with low silver content. High quality alloys are used to produce jewelry. They are quite flexible and go well with colored stones, pearls and enamel.
What metals are used in jewelry
Precious metal alloys for jewelry are strictly marked, and their quality is monitored at the state level. But to make jewelry, various metals are used, decorating them with silver and gold plating or semi-precious stones. The cost of such products is much less, so they are often purchased by young people for a daily change of style.
The main metal “mixtures” used to create designer jewelry include:
- Cupronickel is an alloy of copper, nickel, manganese and iron. The unique composition was known to jewelers back in the 3rd century BC, and it was then called “white copper.” Most often, unique dishes and bracelets are made from this metal.
- Bronze is made from a mixture of tin and copper. Such glory was in demand during the Bronze Age. It was during that period that weapons, jewelry, dishes and household items were poured into their fusion. Modern bronze alloys are high-tech materials that are “diluted” with zinc, nickel, aluminum, phosphorus and other components to obtain unique properties.
- Nickel silver is an alloy of copper, nickel and zinc. A large amount of nickel gives a beautiful white color, somewhat reminiscent of natural silver. Often, state awards and medals are made from this material. It is also often used to produce beautiful jewelry filigree and enamel.
- Pewter is a tin-based material. Jewelry made from this metal does not contain lead or nickel and therefore does not cause allergic reactions. The alloy is ideal for casting and is easy to process. The material was first discovered in an Egyptian tomb whose founding dates back to 1450 BC.
- Brass is a popular material for creating jewelry and plumbing products. It is obtained by a mixture of copper, zinc and auxiliary components. Brass was already known in Ancient Rome.
To make costume jewelry look like items made from a precious alloy, a special coating (galvanic) is applied to it. This is a common method of gilding, allowing the use of a minimum amount of precious metals to create beauty and shine.
Silver samples
The Russian Federation has adopted the metric system of samples. Metric fineness is the number of milligrams of the base noble metal that one gram of the alloy contains.
For example, in a 925 silver alloy, there are 925 milligrams of silver per gram. For simplicity, we can assume that at 925 purity the alloy contains 92.5% silver.
- 800. An alloy with a high copper content, which is why it has a yellowish tint. Suitable for cutlery.
- 830. Properties are identical to the 800th sample. Used for decorative decorations.
- 875. It is used in the industrial production of jewelry and household products - for example, pens.
- 916. Used for the production of tableware items coated with enamel.
- 925. In color and anti-corrosion properties it does not differ from pure silver. This alloy is widely used for making jewelry.
- 960. Used for making filigree products.
- 999. Suitable for storing metal in ingots and silvering, that is, covering jewelry with a thin layer of silver to protect and improve decorative properties.
In jewelry production, 875 and 925 are the most common. Modern Russian GOST 30649-99 describes five grades of silver-based alloys. In all, the alloy is copper.
History of discovery
Silver has been known to people for a long time. Historians first noted its significance around the 4th millennium BC. e. Then the first jewelry appeared from this noble metal, which was used as exchange currency.
Over time, people learned the properties of this material and began to use it to purify water from impurities. Shamans and clairvoyants valued this metal, calling it a protector of the soul. Ritual knives and sacred weapons were made from it. Astrologers called this material moonstone. He was supposed to show the way to lost travelers, drive out evil spirits and darkness along the way.
Coatings of silver products
Currently, the most technologically advanced types and methods of coating silver products are:
- “White” boiling is boiling in sulfuric or hydrochloric acid to reduce the content of copper reacting with hydrogen sulfide in the thin surface layer of the product. The surface acquires a matte tint.
- Electroplating — applying a thin layer of precious metal using the electroplating method. Currently, electroplating is the most popular way to protect and decorate silver jewelry. Depending on the metal that performs the function of the protective layer, the following types of coatings are used:
- Silvering is a coating with a thin layer of pure silver, which reacts weaker than alloys with hydrogen sulfide in the air.
- Rhodium plating is coating with a layer of white and, less commonly, black or yellow rhodium. Rhodium is a precious platinum group metal. It is wear-resistant, does not darken, and has a beautiful mirror-like light-steel shine, which is why in the last 10–15 years it has become one of the most popular coatings on the jewelry market. If repaired by soldering, the rhodium plating turns black and cracks. In this case, the old coating must be removed and a new one applied.
- Gilding is gold plating. It is actively used for complete or partial decoration of silver products.
- Passivation (passivation) — transfer of the top layer of the alloy into a passive state, which sharply slows down corrosion processes. Performs a purely protective function.
- Oxidation is a type of passivation, the creation on the surface of a metal of a dense film of oxides that protects it from corrosion. It is both a decorative and protective coating method.
- Electrophoresis deposition - coating with organic polyurethane foam or acrylic by immersion in an aqueous solution of epoxy or acrylic resin under the influence of tension. Performs only a protective function.
How to care for silver items?
Rings, earrings and chains made of silver can retain their original appearance for decades. To do this, just follow simple recommendations:
- remove jewelry before washing dishes, showering, or other water procedures;
- periodically wipe the product with a soft flannel cloth;
- Rinse with running water approximately once every 1-2 months;
- Do not store jewelry on holders - air accelerates darkening. It is better to use a box or bag made of thick fabric for storage.
If the product does turn black, you can restore its beauty with a variety of available means - from ammonia to lipstick. If you are superstitious, then silver jewelry will have to be periodically energetically cleaned to remove the negativity that has accumulated in them.
Application
Jewelry, watches, tableware, interior decorations, writing instruments, and decorative elements of weapons are made from silver. Silver can be combined with gold, enamel, niello, precious and semi-precious stones, pearls, corals, and ivory. The metal is widely in demand in the chemical industry, in the production of mirrors, and also as a protective and decorative coating. High thermal and electrical conductivity make silver useful in the production of electronics and electrical equipment.
Myths about silver
- Fake silver turns black, but real (old, high-quality) silver does not turn black
A fake can also turn black for a variety of reasons, but if you carefully read our article, you realized that the formation of a thin layer of silver oxide or sulfide is normal for this metal; this is its chemical feature. And in old jewelry, not copper, but palladium and platinum were used as ligatures. This alloy did not really darken, but it was much more expensive. It's chemistry, nothing personal.
- Silver disinfects
This is true, silver has bacteriostatic properties, slowing down the development of bacteria, and a bactericidal effect, killing bacteria with the help of silver ions. But this manifests itself only in concentration, which can be harmful for you and me, because silver is a heavy metal, it is deposited in the body and can cause poisoning. It is safe to wear, but we do not recommend eating it.
You can also remove spoons, rings and pendants from the water: in this case, so few silver ions are released that they are not able to disinfect anything. This also means you don't have to worry about silverware and cutlery. If you use a special ionizer, there will be an effect, but with it it is easy to exceed the permissible concentration and again get poisoned.
If you are reading in English, here is a WHO report on the use of silver for disinfection, which generally repeats the above conclusions and speaks of the lack of information for far-reaching conclusions in most studies on this topic.
- Silver drives away evil spirits
We invite you to check this myth yourself. Find a vampire and stab him with a silver sword, but take precautions: if the sword is rhodium-plated, the effect may be disappointing.
APPENDIX D (for reference). Properties of palladium-based alloys
APPENDIX D (for reference)
Table D.1
| Brand | Estimated density, g/cm | Melting point (range), °C | Hardness HV*, kgf/mm | |
| Solid | Soft | |||
| PdSrN 500−450 | 11,16 | 1200−1210 | 330 | 160 |
| PdSrKrTsM 500−305−0.8−0.3 | 10,20 | 915−1050 | 220 | — |
| PdSrN 850−130 | 11,83 | 1420−1500 | 235 | 125 |
| PdM 850 | 11,54 | 1360−1415 | 220 | 155 |
| PdKM 850−7.5 | 11,53 | 1360−1390 | 160 | 100 |
| PdKM 850−10 | 11,49 | 1360−1390 | 230 | 130 |
| PdNTsM 850−4-1.5 | 11,43 | 1290−1350 | 150 | 90 |
| PdNTsinM 850−3.5−1-0.5 | 11,45 | 1280−1340 | 150 | 85 |
| PdMKr 850−12 | 10,72 | 820−1125 | 310−350 | — |
| PdSr 900−100 | 11,98 | 1510−1520 | — | 55 |
| PdI 900−100 | 12,74 | 1590−1690 | 150 | 90 |
| PdInM 900−5 | 11,40 | 1450−1500 | 170 | 80 |
| PdKM 900−5 | 11,73 | 1370−1400 | 160 | 90 |
| PdNTsinM 900−4.5−2-1 | 11,52 | 1325−1385 | 160 | — |
| PdNTsinM 900−3.5−0.8−0.5 | 11,57 | 1320−1380 | 140 | 75 |
| PdMKr 900−8.5 | 11,22 | 820−1250 | 220−240 | — |
| PdI 950−50 | 12,44 | 1560−1610 | — | 65 |
| PdRu 950−50 | 12,16 | 1560−1580 | 280 | 120 |
| PdSrM 950−25 | 12,02 | 1500−1520 | — | 95 |
| PdSrKGa 950−35−0.8 | 12,00 | 1310−1330 | 178 | — |
| PdRe 950 | 12,42 | 1555−1565 | 250 | 90 |
| PdInM 950−2.5 | 11,92 | 1290−1340 | — | 70 |
| PdKM 950−3 | 11,95 | 1450−1480 | 195 | 80 |
| PdKin 950−4 | 11,93 | 1290−1310 | 219 | — |
| PdNTsinM 950−2-0.5−0.5 | 11,95 | 1335−1410 | 165 | — |
| PdMKr 950−3 | 11,45 | 820−1200 | 300−320 | — |
| PdRuIt 990−5 | 12,11 | 1550−1560 | 199 | 120 |
| PdTsrKh 990−0.7 | 12,01 | 1550−1600 | 200 | 108 |
| PdNH 990−0.6 | 12,08 | 1500−1550 | 190 | 130 |
| PdVit 990−0.5 | 12,08 | 1470−1550 | 200 | 90 |
| *Hardness was determined on samples with a degree of deformation of 75%-95%. | ||||
Table D. 1. (Changed edition, Amendment No. 2).