Today, ultraviolet light is used to solve a wide variety of problems, including the search for minerals. When exposed to ultraviolet light, various minerals begin to fluoresce, that is, they glow in different colors and shades. Thanks to this, in the dark it is very easy to distinguish a noble stone from ordinary gray stones. This effect is actively used not only directly to search for minerals and ores, but also to study them: determining the purity of stones, as well as the presence of various impurities in their composition.
Story
Vases, bowls, and other dishes that glow under the rays of the sun were made back in Ancient Greece.
There these products were available only to the nobility. Medieval alchemists, conducting experiments on fluorite crystals, noticed that when heated they began to glow in the dark. Often such tests ended in an explosion, and the experimenters were poisoned by poisonous gases. For this, the mineral was nicknamed the devil's stone.
Fluorite has other names:
- ore flower;
- South African emerald;
- fluorspar;
- Murin.
In 1529, the founder of mineralogy, the German scientist Gregory Agricola, came up with the name “fluores” for the stone because of its fusibility (translated from Latin as “flow”, “fluid”). Then the name “fluorite” was assigned to the mineral.
From the name of the stone comes the word “fluorescence,” which refers to the glowing effect under ultraviolet rays or other light sources.
Fluorescence effect
Fluorescence is the optical effect of precious stones, which is expressed in their special glow. This happens as a result of exposure to ultraviolet light or heating.
Why do stones glow?
Fluorescence is a visible reflection of chemical processes taking place inside a substance. Under the influence of an external energy source: light or thermal waves, the electrons of atoms transition to a different energy level. Which in turn leads to changes in light parameters.
When was the glow of stones first discovered?
The phenomenon was discovered by the English physicist D. G. Stokes while studying fluorite in 1852. The name is suffixed with “essence,” which is Latin for “weak action.” Subsequently, the term began to be used in relation to gems, to describe their specific characteristics.
What are the types?
According to the degree of intensity, fluorescence can be of several types:
- Zero;
- Weakened;
- Moderate;
- Strong.
from left to right 4 types of fluorescence
Physicochemical characteristics
Fluorite is a unique mineral in that its formula contains fluorine. From the point of view of chemists, it is calcium fluoride.
- The chemical formula is CaF2 .
- Color - blue, purple, red, blue-green, yellow, brownish-yellow, white; happens to be colorless.
- The shine is glassy.
- Transparency - transparent, translucent.
- Hardness - 4.
- Density - 3.18 g / cm3 .
The mineral is fragile and soluble in hydrochloric acid (this releases toxic gas).
It glows under ultraviolet light. If you heat it up, it can glow in the dark.
The heated mineral becomes discolored. When irradiated with X-rays, the color is restored.
Some samples are radioactive, but do not pose a serious danger.
THE HIDDEN RAINBOW OF STONE
[Historical version of this article from 1999] [Luminescence at the GeoShow in St. Petersburg] [History of the exhibition “Luminescence of Minerals”] [About the exhibition-fair “World of Stone”]
Imagine the light that streams from the stone... What picture came to your imagination? Surely, this is a beautiful gemstone, a diamond or a ruby, cut by a master’s hand and shimmering with all the colors of the rainbow hidden in the light of the sun. Or maybe it’s a noble opal, the radiance of which seems to break through the opaque stone shell. But the sun will disappear and the beautiful stones will fade, they will all become equally gray. Few people know that the time is coming to awaken its true light in a stone, not the reflection of other people’s rays, but its own rainbow, hidden from human eyes.
| Zircon (yellow), microcline (purple-red), calcite (pink), fluorite (3 crystals in foreground); HF UV |
In front of me in the darkness lies a scattering of luminous stones. All the colors imaginable are collected together - here are pink and bright red, like the coals of a smoldering fire, calcite crystals, next to them are soft blue, blue lights of fluorite, zircon shining with gold, white aragonite. What made this “gravel”, inconspicuous in the light of the sun, suddenly shine in the darkness? There is only one solution - ultraviolet light, radiation from special lamps, invisible to the human eye, but making stones sparkle. This wonderful phenomenon is called luminescence
, or
fluorescence
. It has not been known to humanity for so long - only since the first source of ultraviolet light was created in the last century. And its name was given by the mineral fluorite, one of the brightest stone “night flowers”, by no means a precious stone that is easy to find in almost all corners of the world.
few people know that under ultraviolet light even “simple” stones can shine with all the colors of the rainbow
TWO WORDS ABOUT ULTRAVIOLET
To see luminescence, a person needs a special device that emits ultraviolet radiation. However, there is nothing mysterious about this radiation - it surrounds a person all day long while the sun is burning in the sky. But we cannot use it to search for luminescent minerals - together with the “solar” ultraviolet, a stream of ordinary, visible light pours on us, which is too bright and drowns out the weak glow of minerals. If we sort the solar radiation incident on the Earth according to the energy of the light waves, we get a spectrum
, the central part of which will be occupied by visible light, in which all the colors of the rainbow alternate. On the one hand, visible light is limited to the region of infrared radiation, which is perceived by humans as heat. On the other side, “behind” the violet color, there is a wide zone of ultraviolet radiation. The shorter the wavelength of light, the greater the energy of a light particle (photon). Ultraviolet radiation (often referred to as "UV" for simplicity) has a wavelength of 100 to 380 nm and has an energy much greater than that of visible light. But even in the UV region itself, there are ranges of long-, medium-, short-wave and vacuum (“far”) ultraviolet (doesn’t it resemble radio wave ranges?).
| Ultraviolet rays in the spectrum of electromagnetic radiation (LW - long-wave, SW - medium-wave, SW - short-wave). The wavelength is indicated in meters, and on the detailed scale for the UV region - in nanometers (1 nm = 10-9m). |
Not all UV radiation is harmless to humans. Only long-wave UV is relatively safe, which causes tanning on the skin and, within reasonable limits, is even good for health. It is used in medical lamps, solariums, discos, and also to detect counterfeit money and documents. But shorter wavelength types of UV can cause damage to the fundus of the eye and skin cancer and are used only for technical purposes with special precautions. Fortunately, the most high-energy ultraviolet radiation emitted by the Sun is retained high in the atmosphere by the ozone layer (which is why humanity has been so concerned about the problem of “ozone holes” in recent years). Those readers who are keen on searching for luminescent minerals will like the fact that all ultraviolet, except long-wavelength, is blocked by ordinary glass, that is, ordinary glasses are enough to protect against it (this does not apply to the radiation of special powerful sources of UV radiation used in science and technology, well, they won’t bother you and me). But for the same reason, if you try to irradiate a stone through glass, no luminescence will occur!
| Plaster double; DV UV |
To protect against ultraviolet radiation, a regular sunglasses or glasses are sufficient
BIRTH OF A RAINBOW
Now let's imagine what happens if ultraviolet radiation hits the crystal. “Sentries of atoms” - electrons located in the outer electron shells will absorb UV radiation quanta and for the shortest time (millionths or billionths of a second) will move to a higher energy level, to an “excited state”. This state is unstable, and soon the electrons will distribute part of the energy to the crystal lattice in the form of thermal vibrations (i.e., the crystal will simply heat up a little), and part of the energy (“if you’re lucky”) will be emitted into the surrounding space in the form of light. solid state physics can predict whether we will be “lucky” or whether luminescence will not occur.
. The processes accompanying luminescence are very complex and depend on the type of crystalline substance, impurities and other “defects” in its structure, ambient temperature and other factors.
| This is how luminescence arises: at the first stage (A), an electron (1) absorbs a quantum of ultraviolet radiation (2), which transfers it to a higher energy level (3); then (B), having transferred part of the energy to the crystal in the form of heat (4), the electron returns to the original level (5), emitting the remaining excess energy into the surrounding space in the form of light (6). |
Now it’s clear why ultraviolet radiation (and not infrared, for example) is required to observe luminescence - if we want to see
luminescence (after all, there is luminescence and not only in the range of visible light), then to excite it we need radiation with greater energy than visible light. However, in a scientific laboratory, for the same purpose, we can use other sources of excitation - X-rays and electron beams, and the glow of the mineral will become even brighter. Such devices are inaccessible to an amateur - they are too bulky, very expensive and require exceptional precautions. We have at your disposal ultraviolet lamps that contain mercury vapor under pressure. When electrical current is passed through, they emit visible light and ultraviolet radiation. If the lamp is covered with a special light filter that blocks visible light and allows ultraviolet light to pass through, we will get the simplest device with which we can go out in search of “firefly minerals.”
The more powerful the lamp, the more photons of UV radiation per unit surface area of the mineral, the brighter the luminescence. Portable UV lamps usually have a power of 4-6 W, but if you want to create a small museum or showcase with luminescent minerals at home, then you should purchase special display lamps with a power of 15-30 W.
| Aragonite: HF UV (left) and DV UV (right) |
You should not think that if a mineral luminesces, then this can be detected using any ultraviolet source. As is known, an electron belonging to an atom or crystal does not change its energy arbitrarily, but in “quanta”, that is, in a stepwise manner. This means that it is possible to transfer an electron to a certain energy level only by exposing it to radiation with a certain wavelength. And if there is no such wavelength in the spectrum of the lamp, then we will not see luminescence. True, one must keep in mind that often a crystal has a whole set of possible electronic levels, each of which is “responsible” for its share of luminescence and “catches” ultraviolet radiation of “its own” strictly defined energy. Here lies another remarkable feature of luminescence: the energy that electrons emit when returning from different electronic levels corresponds to different parts of the spectrum. So by changing the wavelength of UV light, you can sometimes make a mineral glow a different color. Of course, the specific features of the glow will depend on the type of mineral and the nature of the impurities in it.
the greater the power of the ultraviolet lamp, the brighter the luminescence of the mineral, and the wavelength of the UV radiation determines whether luminescence will occur and what its color will be
In practice, when studying luminescence, specialists select ultraviolet light of the required wavelength by selecting light filters or other methods. Portable lamps are manufactured with built-in light filters, and lamps are usually produced that emit in two standard ranges - 254 nm (“short-wave ultraviolet”, HF UV) and 366 nm (“long-wave ultraviolet”, LW UV). Some lamps have two different light filters built in at the same time. Such standardization makes it possible to use lamps from different manufacturers and obtain comparable results, which is especially important when using literary sources.
So, the mystery of the glow of minerals has been partially solved. It's time to descend from the heavens of theory onto the rocky Earth.
FIREFLY STONES
The attentive reader should have noted that a variety of minerals can luminesce. Among them are precious stones - diamond, ruby, sapphire, topaz, spinel, kunzite, opal, lapis lazuli, turquoise, amber, but there are also “simple” minerals that will never attract your eye if they catch your eye not as crystals, but in the form of debris. Meanwhile, it is not difficult to find precisely such minerals, and the joy at the sight of a glowing stone is in no way inferior to the feeling evoked by a jewelry stone in a precious setting. More than 3,000 minerals have already been discovered in nature, and more than 500 of them luminesce. This means that almost any rock can contain a luminescent grain. The most famous luminescent minerals are fluorite, carbonate minerals (calcite, dolomite, magnesite, aragonite and others), apatite, zircon, and scheelite. Ore minerals are native metals; their oxides and sulfides usually do not luminesce. In addition, unfortunately, such beautiful minerals as quartz, chalcedony and their varieties, as well as many beryls, garnets, and tourmalines, do not “glow.”
Many “simple” minerals luminesce, which are easy to find yourself or buy at an exhibition
Typically, minerals do not glow “in their pure form”; for luminescence to occur, the presence of activators
— structural impurities of various chemical elements. Typical activators are rare earth elements, chromium, manganese, uranium. Luminescence can be caused by transition metal complexes, molecular ions, and even defects in the crystal structure of a mineral that are not associated with impurities.
| Sheelite; HF UV | Calcite; HF UV | Calcite; DV UV |
Here are short descriptions of the luminescence of some common minerals (indicated: the name of the mineral, its chemical formula, luminescence colors in the HF and LW ranges):
Diamond.
C. HF and DV: blue, light green, yellow, orange, red.
Aragonite.
Ca[CO3].
HF and DV: white, green, yellow, cream, bluish-white, red. orange. Apatite.
Ca5[(F,Cl,OH)|(PO4)3].
HF and DV: orange, yellow. brown, red, cream, white, purple, bluish-gray. Calcite.
Ca[CO3].
HF and DV: red, white. green. blue, orange, violet, purple. Corundum (ruby, sapphire).
Al2O3.
HF and DV: red, magenta, orange, yellow; KV: blue. Fluorite.
CaF2.
HF and DV: blue, violet, white, red, yellow, cream. Zircon.
Zr[SiO4].
HF (weaker in the Far East): bright yellow, orange. Scheelit.
Ca[WO4].
KV: white-blue, cream, yellow. Spinel.
MgAl2O4.
HF and DV: red; DV: green, blue. Uranium micas (otenite, torbernite).
(Ca,Cu)[UO2|PO4]2·10-12H2O. HF and DV: bright yellow-green, green, yellow.
| Opal: HF UV (left) and DV UV (right) |
There is another interesting phenomenon that you can discover: some stones, after you turn off the UV lamp, go out slowly, as if fading. This phenomenon is called phosphorescence
, it is often observed in opal, calcite, and aragonite. That is why, even from one mineral, if you try, you can create a wonderful collection in which all samples will glow in their own way, surprising and delighting you and your guests.
IS THERE A BENEFIT FROM LUMINESCENCE OF MINERALS?
Before rushing in search of beautiful samples, I would like to say that the luminescence of minerals is not only a beautiful and funny phenomenon, but also a useful tool in a variety of areas of human activity. First of all, man began to use luminescent minerals to search for deposits of rare metals. Having a small ultraviolet lamp on hand, it is easy to diagnose minerals such as scheelite (the most valuable tungsten ore), zircon (zirconium), and many uranium minerals. At a time when the luminescent method was virtually the only available method for field diagnostics of these minerals, even special manuals on their determination were published. And today, at some tungsten deposits, miners have an additional ultraviolet lamp built into the miner’s flashlight.
Another important task is to identify a fake gemstone and distinguish a natural stone from a synthetic one. A mistake here can be costly, so gemologists
- specialists in precious stones have been using ultraviolet lamps for a long time, we owe them the introduction of standardization of lamps according to the lengths of UV radiation.
In science and technology, the luminescence of natural and synthetic minerals is also not in last place. The study of the laws of formation of deposits in the earth's crust, the characteristics of the growth of minerals, and the properties of synthetic materials cannot be done without luminescent instruments. However, it is impossible to talk about all the areas of application of luminescence and all its varieties in a few words.
HOW TO COLLECT A COLLECTION OF GLOWING STONES
So what should a beginning collector of luminescent minerals stock up on? The only tool needed is an ultraviolet lamp. True, this is where the problems begin: where can I get a portable UV lamp in Russia? It is difficult to find domestic manufacturers of such lamps; unfortunately, they are not known to the author of this article. Such enterprises exist abroad, but it is difficult to contact them. There are several ways out - if possible, it is worth visiting a mineral exhibition or a specialized store abroad; you can find relevant companies on the Internet or in magazines for mineral collectors (Lapis, World of Stone, etc.). It is not harmful to look at exhibitions and sales of semi-precious stones (for example, at the regular exhibition “World of Stone” in St. Petersburg). Those who are persistent will get lucky sooner or later.
everything a beginning luminescent mineral collector needs: a portable ultraviolet lamp with two standard radiation ranges, safety glasses, a flashlight, a geological hammer, a backpack and patience
It’s worth sparing no expense and buying a lamp that emits in both the short- and long-wave ranges. In addition, it would be good to purchase special safety glasses - God protects those who are careful, although the power of our lamps is low enough to cause real damage to health (glasses wearers can rejoice - ultraviolet radiation does not pass through glass lenses).
You can start looking for “luminous stones” at the same exhibition and sale of minerals. Here you may be lucky if you come across a cheap and inconspicuous-looking stone (for example, one of the carbonates) that shines with a bright light. Remember that luminescence will not be visible in bright light - you need to take refuge in a dark room or at least cover your head with a thick jacket. Those who like to find everything on their own cannot do without a geological hammer, a flashlight, a backpack and a lot of patience. Most likely, the hunt will be at night, so a flashlight is absolutely necessary. Be careful, night is not the best time for walking, so when going out into an old quarry or “out into nature” in search of rock outcrops, take the necessary precautions!
| Magnesite (white), calcite (orange-red), fluorite (3 crystals in foreground); DV UV |
Where to go? There is no universal answer to this question. Of course, there are wonderful deposits where there are especially many luminescent minerals, but this is a topic for another conversation. The main thing is that near your home, on the river bank, in rocky cliffs or in a mine, you can find wonderful specimens worthy of the most serious museum collection.
WHAT TO READ (for the most curious)
I read a small chapter on the luminescence of minerals, but I still have many questions. Where can I go for further information? Unfortunately, popular books on the luminescence of minerals, the number of which has already exceeded a dozen abroad, are not available in Russian. But on it you can find excellent scientific literature that has no foreign analogues, which can be useful to those who want to better understand the physics of the luminescence phenomenon. A natural paradox... Let it help us both to become fully acquainted with the subject and to learn English better! Both can be useful, since a lot of modern information about the luminescence of minerals is available on the Internet computer network (and all on foreign networks): here are photographs of luminescent minerals, and popular information, scientific and amateur societies (and this, it turns out, available abroad), the opportunity to order a book and simply meet lovers of luminescence and everything mysterious and beautiful that surrounds the wonderful world of minerals.
Literature
Marfunin A.S.
Spectroscopy, luminescence and radiation centers in minerals.
M., Nedra, 1975. Solodova Yu.P.
and others. Determinant of jewelry and semi-precious stones.
M., Nedra, 1985. Tarashchan A.N.
Luminescence of minerals.
Kiev, Naukova Dumka, 1978. Robbins, M.
The collector's book of fluorescent minerals.
New-York, Van Nostrand Reinhold, 1983. Robbins, M.
Fluorescence: Gems and minerals under ultraviolet light.
Phoenix, Arizona Geoscience Press, 1994. Warren, T. S. et al.
Ultraviolet light and fluorescent minerals: Understanding, collecting and displaying fluorescent minerals. Thomas S. Warren Publ., 1995.
The article was first published on the website of the Department of Mineralogy, Crystallography and Petrography of the St. Petersburg Mining Institute in 1999. Original article...
A version of the article “The Hidden Rainbow of Stone” was published in the 3rd issue of the magazine “Mineral” (1999, N2).
A version of the article was published in the collection of popular science articles of the Club of Young Geologists of the St. Petersburg House of Youth Creativity.
The author expresses his deep gratitude for the samples provided to Professor of the St. Petersburg State Mining Institute (Technical University) Doctor of Geological and Mineralogical Sciences M.A. Ivanov (fluorites, scheelite) and Associate Professor Candidates of Geological and Mineralogical Sciences V.A. Romanov (calcite, aragonite , opal, gypsum) and V.V. Smolensky (zircon, feldspar, calcite), as well as special thanks to the senior laboratory assistant of the Department of Mineralogy, Crystallography and Petrography of St. Petersburg State Hydrological Institute (TU) T.V. Spetsova.
Varieties
Fluorite, based on its structure and color, was divided into the following varieties:
- Alpino. Mined in the Swiss Alps. Octahedral crystals have a red color scheme.
- Antozonite (radiofluorite, fluorspar). Radioactive stone of dark purple color. When cracked, it releases a specific odor.
- Derbyshire John (Derbyshire spar, blue John, blue John). It is mined in Derbyshire (UK). Blue colored stones. The structure is usually banded: blue, purple or cyan stripes alternate with greyish, white, yellow.
- Lithos-lazuli . It is distinguished by concentric stripes of red (purple) color.
- Ratovkit . Loose stones of purple color. The origin is sedimentary.
- Chlorophane . Instances that exhibit an intense green fluorescence effect when heated.
Sometimes calcium in fluorite is replaced by other elements. These types of stone are:
- Yttrofluorite (calcium replaced by yttrium).
- Zerfluorite (contains cerium instead of calcium).
Fluorite
Fluorspar is the mineralogical name for fluorite. The stone got its name back in the 16th century due to the fluidity property it possesses. Fluor is translated from Latin as “fluid.” And the “father” of mineralogy, George Agricola, awarded him this name.
But this does not mean at all that the scientist discovered this stone. The mineral fluorite has been used by humanity for many centuries. In ancient Rome, vases made from this mineral were highly valued. But then he was called differently - murin.
And the cost of such things was commensurate with the price of gold items. None of the vases have survived; we can judge their beauty only from descriptions in the works of Pliny the Elder. In Saxony, miners called this stone the ore flower for its unusual color. Objects and jewelry found in the Czech Republic and Ukraine are more than 100 centuries old.
There are many deposits of this mineral. The largest are located in Germany, Scotland, England, China, and the USA. In Russia, it is mined in the Primorsky Territory, Buryatia and Transbaikalia. The most important export suppliers are Mongolia, China and Kazakhstan.
Varieties and physicochemical properties
Fluorite stone can be very diverse. It is impossible to choose a pair of identical stones or even very similar ones - they are all different. Colors range from completely colorless to black. It has a heterogeneous color, with transitions from dark (along the edges) to light (in the center). There may be spots, stripes and stains with a smooth flow of colors.
The most common stones are blue and purple. Yellow, red and green fluorite are often found. And transparent and colorless ones are very rare. The color is directly dependent on the content of rare earth elements, as well as the location of extraction.
Based on the presence of impurities and color, the following groups are distinguished:
- Ratovkit. Color ranges from purple to violet. Fine-earthy, granular and loose structure.
- Yttrofluorite. Enriched with yttrium. Has yellow tints.
- Chlorophane. It contains samarium ions. It is characterized by a green color, which becomes brighter and more saturated as the temperature rises.
- Antozonite. Contains fluoride. Radioactive. Typically dark purple, sometimes almost black. The so-called stinking spar, or Derbyshire amethyst. When crushed or sharply struck, it emits a characteristic fluoride aroma.
- "Blue John" A dense, grainy gem (mined in Devonshire). Two-color striped mineral. Purple with white or yellow.
- Zerfluorite. Enriched with elements of the cerium group.
Crystals most often have a cubic shape, sometimes octahedral, and even more rarely rhombic dodecahedron. They can be single, binary (in the form of two interpenetrating cubes) and aggregate (several crystals and their intergrowths). They have a greasy, glassy sheen.
This mineral belongs to the group of calcium fluorides. Its chemical formula is CaF2. The properties of fluorite stone include the following qualities:
- It dissolves in acids, in particular hydrochloric acid, releasing toxic hydrogen sulfide.
- Melting point above 1360 °C.
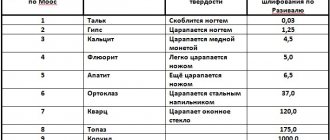
- The hardness on the Mohs scale is 4. It scratches very easily.
- When exposed to ultraviolet rays it begins to glow.
- When heated, it changes the density of its internal color and glows in the dark.
- Changes under radioactive radiation.
- Increased fragility. Upon impact, it shatters into pieces.
Application
Fluorite can be called a hard worker stone. Most minerals are mined specifically for production. The use of fluorite in industry is very wide:
- In the production of hydrofluoric acid and anhydrous hydrogen fluoride. For further production of complex fluoride compounds.
- As a flux in the metallurgical industry. Accelerates the melting process, improves slag separation, and lowers the melting point.
- As additives in welding electrodes. They improve the quality and strength of welds.
- In the nuclear industry.
- In ceramic production. In the manufacture of enamels and glazes.
- In the glass industry. To add color to glass and reduce melting time. For making lenses.
- In the production of aluminum. Cryolite, which also contains fluorspar, is used in production.
- In the cement industry. Reduces firing temperature, improves cement quality.
In the jewelry industry, fluorite is practically not used as inserts, although it is considered semi-precious. Beads, bracelets, pendants, etc. are made from it. It is used as an ornamental stone for making figurines, boxes, vases and various souvenirs.
It is of interest to collectors because of its very beautiful, unusual and large crystals. Since ancient times, fluorite has been passed off as real precious stones, such as ruby, amethyst, citrine, and emerald. But it is easily recognized due to its low hardness
Magic properties
They say that it surpasses all stones in its magical qualities, as it glows in the dark. He is considered the most powerful in esoteric terms. It can dramatically change the course of a person’s life, so it must be used wisely. The following magical properties of fluorite can be distinguished:
- opens the “third eye”;
- with its help they look at the future during spiritualistic seances;
- gives the gift of providence;
- gives wisdom;
- improves intuition;
- protects from ill-wishers.
According to the horoscope, fluorspar corresponds most to Aquarius, and to a lesser extent to Pisces and Gemini. Those signs for whom this stone is suitable will receive great energy supply and help from it.
The most valuable is a transparent, colorless mineral. Its energy is beneficial to all zodiac signs. But there is one exception. Fluorite should not be worn by Sagittarius. In them it causes loss of strength, fear and depression.
The crystal is a powerful stimulator of spiritual development, because it distracts from material values and vanity. It is recommended to store it in a tight box or box. It is believed that in the dark the stone accumulates power.
Ancient magicians cherished their talismans like the apple of their eye. A purple pyramid-shaped stone is used for meditation, as it helps to tune into subtle planes. It helps to gain clarity of mind, wisdom and peace of mind.
Medicinal properties
But this stone is used not only in magic. It is also successfully used for medical purposes. It is not used internally (in powder form), since fluoride is harmful to health. It is used in lithotherapy in the form of massage balls for a general restorative massage of the head and body. A number of diseases are treated by applying warm stones. Let's list how fluorite helps:
- relieves headaches;
- normalizes blood pressure;
- improves sleep;
- improves brain activity, relieves epilepsy attacks;
- strengthens the cardiovascular system;
- helps to get out of depression.
Gives energy and strength, relieves stress and fatigue. Calms the nervous system and is highly recommended for people prone to hysterical conditions. Helps with weather dependence.
Transparent fluorspar harmonizes the aura and energizes. Green crystals cleanse energy, heal mental wounds, open the subconscious and enhance intuition. Yellow stones are suitable for creative people; they help unlock potential.
Blue fluorite organizes brain activity and manages energy. The violet or purple mineral is the most mysterious and magical, since it is it that opens the “third eye” and gives common sense.
Fluorite's properties are a powerful talisman and amulet. It is suitable for everyone, not just magicians and soothsayers. It is useful for lovers to have it to protect them from the evil eye; in the family it will protect them from quarrels.
This stone helps lonely people find their soulmate. It will protect you from misfortunes and help you overcome difficulties. It is very useful to carry a fluorite product with you. It could be a ring, earrings or just a keychain.
Application
Fluorite is a stone used in many fields.
Jewelry
Thanks to its beauty and rich color range, this stone is popular among jewelers.
Jewelry (beads, necklaces, bracelets and sets) made from pieces of different colors look especially impressive.
The frame is selected so that it harmonizes with the color of the stone: cupronickel, silver, “golden” jewelry alloy, irradiated brass.
Arts and crafts
The mineral is malleable in processing. Tableware, souvenirs, and small plastic items are made from it. The bonbonnieres, photo frames and other little things decorated with it are not cheap, so they can be classified as VIP gifts.
They make pyramids, balls, and body amulets from fluorite crystals.
Other areas
The industry uses raw materials of non-jewelry quality:
- Metallurgy. The addition of fluorite lowers the melting point of the ore.
- Chemical industry. Fluorspar is used to make hydrofluoric acid.
- Optics. Colorless transparent specimens are used to make lenses for cameras and night vision devices.
Collectors of mineralogical collections are offered a wide range of this stone:
- agglomerates;
- Druze;
- crystals of various shades and deposits.
Description and types of luminous stones
Glowstones are not a type of natural mineral. They are made specifically for decorating a room or garden plot. Of course, there are minerals that have luminescence, but it is only visible under ultraviolet light. And it is expensive to use such stones as a decorative material.
Fluorescent stones are made in several ways:
- A simple and cheap option is to coat regular pebbles or cobblestones with fluorescent paints. It’s easy to make pebbles yourself, giving free rein to your imagination. They will serve for a long time, you just need to periodically renew the paint.
- Plastic stones with phosphor catalyst. This is already an industrial version. Plastic is mixed with a special chemical composition and cobblestones of different shapes and sizes are made from it. During the day, the composition accumulates sunlight, and in the darkness of the night it releases it. This decoration will work for about 20 years.
- Polymer pebbles with LEDs. Hollow boulders are made from polymer material, and LEDs are placed inside. They look incomparably better than painted stones or cobblestones with a catalyst. However, their service life is short. In addition, LEDs will not work in severe frost.
Painted pebbles or products with a catalyst are suitable for the garden. Polymer pebbles with LEDs are best used for decorating rooms.
Watch the video to see how the stones look on the site day and night:
Medicinal properties
Fluorite, according to lithotherapists, helps to heal the body.
When using this stone for medicinal purposes, you should not refuse treatment prescribed by your doctor.
The healing properties of fluorite are used if there are the following problems or diseases:
- insomnia, depression, tendency to stress;
- atherosclerosis;
- consequences of stroke;
- kidney diseases;
- weather dependence (aches, migraines);
- eye fatigue, weakened vision (it is especially useful for the eyes to look at the light through a transparent green or blue fluorite crystal);
- aging of the skin (you can rejuvenate it with a massage with fluorite balls or rollers);
- weakening of the immune system (wearing fluorite jewelry will help boost it).
This mineral strengthens joints, bones, teeth and accelerates the regeneration of body tissues after injury or surgery.
Industrial production of fluorescent stones
All three types of glowstones are found in industrial production. And if you can paint pebbles with luminous paint yourself, then it is better to buy LED or catalyst products in specialized stores.
Plastic fluorescent cobblestones are made from plastic mixed with a special chemical composition. This composition is capable of accumulating ultraviolet radiation from sunlight and glowing at night. This decoration does not require special recharging, lasts a long time, and is not afraid of water.
Polymer LED stones are round, hollow containers. They are made of polymer material. A set of LEDs and a battery are placed inside. The decoration requires regular recharging and does not work under water or at low temperatures.
Magic properties
Fluorite, in addition to healing properties, also has magical qualities.
The stone helps the owner to discover and develop abilities and talents, to take a fresh look at himself and the people around him.
Fluorite is important for a person who is trying to develop his analytical abilities. It will help highlight the main thing; its owner will not waste time on secondary issues and problems.
This stone is appreciated by fans of meditation. It is believed that by peering into a fluorite ball, one can see future events.
The mineral (especially green fluorite) is an excellent amulet against negative external influences. Attempts by other people to control the consciousness of its owner will be unsuccessful.
Fluorite takes away not only negative mental energy, but also radiation from gadgets. It is useful to place a stone near a computer, microwave, etc.
The color of fluorite affects its magical properties:
- Blue. Helps unlock creative potential and organize thoughts. Depending on the needs of the body of the owner of the stone, it calms or activates its energy.
- Violet. Ideal for meditation, helps open the “third eye” and also gain sanity.
- Green. Neutralizes negative energy directed at the owner of the mineral and helps heal spiritual wounds.
- Yellow. Strengthens creative and intellectual abilities. Positively affects the microclimate in the team.
- Red. Relieves despondency, depression, encourages creative activity. Such a stone is not suitable for overly aggressive people, and it is not recommended for other people to wear it constantly.
- Colorless. Harmonizes the intellectual and spiritual level, the work of all chakras. Charges a person’s aura with the energy of the Universe.
What is a fluorescent mineral?
All minerals have the ability to reflect light. This is what makes them visible to the human eye. Some minerals have an interesting physical property known as "fluorescence."
These substances have the ability to temporarily absorb small amounts of light and instantly release it at a different wavelength. This change in wavelength causes a temporary change in the color of the mineral.
The color changes of fluorescent minerals are most impressive when they are illuminated in the dark with ultraviolet light (invisible to humans).
More about fluorescence
Fluorescence in minerals occurs when a sample is illuminated with a specific wavelength of light. Ultraviolet (UV) light, X-rays, and cathode rays are typical types of light that cause fluorescence.
These types of light have the ability to excite sensitive electrons in the atomic structure of a mineral. The excited electrons temporarily jump to a higher orbit within the mineral's atomic structure.
When these electrons return to their original orbit, a small amount of energy is released in the form of light. This process of releasing light is known as fluorescence.
The wavelength of light released from a fluorescent mineral is often noticeably different from the wavelength of incident light. This leads to a noticeable change in the color of the mineral. The glow continues as long as the mineral is illuminated by light of the appropriate wavelength.
How many minerals glow under UV light?
Most minerals do not have noticeable fluorescence. Only about 15% of minerals have a glow that people can see, and other examples of these minerals will not glow. Fluorescence usually occurs when certain impurities known as "activators" are present in a mineral.
These activators are usually metal cations such as tungsten, molybdenum, lead, boron, titanium, manganese, uranium and chromium. Rare earth elements such as europium, terbium, dysprosium and yttrium are known to contribute to the fluorescence phenomenon. The glow of minerals can also be caused by structural defects in the crystals or organic impurities.
Some impurities quench fluorescence. If iron or copper is present as impurities, they can reduce or eliminate fluorescence. In addition, if the mineral activator is present in large quantities, it may reduce the fluorescence effect.
Most minerals glow in one color. Other minerals have multiple fluorescent colors. Calcite is known to glow in red, blue, white, pink, green and orange.
Some minerals exhibit multiple colors of fluorescence in a single sample. These may be banded minerals that exhibit several stages of growth from initial solutions with varying compositions. Many minerals fluoresce one color under short-wave UV light and a different color under long-wave UV light.
Fluorite: the original "fluorescent mineral"
One of the first people to observe fluorescence in minerals was George Gabriel Stokes in 1852. He noted fluorite's ability to produce a blue glow when illuminated with invisible light "beyond the violet end of the spectrum."
He called this phenomenon "fluorescence." This definition is widely accepted in the fields of mineralogy, gemology, biology, optics, commercial lighting and many other fields.
Most fluorite samples have fairly strong fluorescence. An observer can take them outside, hold them in sunlight, then move them into the shade and see the color of the mineral change. Only a few minerals have this level of fluorescence.
Fluorite typically glows blue-violet under short and long wavelength light. Some samples are known to accumulate cream or white color, but many do not fluoresce. It is assumed that the glow in fluorite is due to the presence of yttrium, europium, samarium or organic material as activators.
Fluorescent geodes?
You may be surprised to learn that geodes (geological formations) have been found with fluorescent minerals inside. Some of the geodes discovered near the community of Dugway, Utah, are filled with chalcedony, which produces a lime-green fluorescence caused by the presence of traces of uranium.
These geodes are amazing for another reason. They formed several million years ago in archaeolite gas pockets. Then, about 20,000 years ago, they were destroyed by wave action along the shoreline of a glacial lake and transported several miles to where they settled in lake sediments. Today, people excavate these geodes and add them to geodetic and fluorescent mineral collections.
Who is suitable according to their zodiac sign?
Fluorite has perfect compatibility with two zodiac signs: Virgo and Gemini :
- Virgo, under the influence of the stone, will begin to think about the meaning of life, highlight the main priorities, without wasting time on unimportant activities.
- Gemini will become more attentive and kinder to others, and will begin to understand those areas of life that they considered uninteresting.
According to the horoscope, the magical properties of fluorite are also suitable for the following zodiac signs:
- Aquarius will get stronger physically, demonstrate their abilities and talents in important matters, and achieve success in their chosen field of activity.
- Aries , striving for spiritual self-improvement, will receive help from this mineral.
- Capricorn will feel the urge to be creative.
- Pisces will be able to recharge their energy from the stone so much that they will happily share their vitality and optimism with other people.
The rest of the zodiac signs (except one) can wear jewelry with this mineral, but its magical properties most likely will not affect them.
- for Sagittarius , as it can contribute to the distortion of any information. The magical properties of the following stones are well suited to Sagittarius: lapis lazuli, apatite, chrysocolla, prasiolite, blue sapphire, spinel, ruby, chalcedony, ulexite, chrysoberyl, rhodolite, diamond, amber, sodalite, blue quartz, variscite.
The advantage of glow-in-the-dark stones
Such decor today attracts site owners for many reasons.
- The backlit decor looks amazing at night.
- Helps navigate space at night.
- Glowing stones for landscape design serve as an additional source of light.
- This is very exotic, it is not found in every yard, which allows you to make your site unique.
Making it yourself will not take much effort and time. And the result will please the owner of the site.
Glowing Stone Decor Ideas
The design of a site using luminous stones is limited only by the designer’s imagination.
Glowing paths
An option that allows you to highlight a path on the site. This is the most commonly used type of decoration. The main advantage is practical use. The path is clearly visible in the dark; there is no risk of accidentally stepping into a flowerbed in the dark. With this design, the entire path is isolated, or stones are placed mainly along the curbs.
Milky Way effect
Crumbs or pebbles are scattered randomly along the path or area. At night it looks like a starry sky on earth.
Framing the flower bed
Large cobblestones are used: they look better. They are placed randomly along the edge of the flowerbed. Cobblestones are placed on the flowerbed itself. Paint manufacturers claim that the composition is completely safe for humans and can even be used indoors. But you shouldn’t use such paints to decorate your garden. When it rains or sprays water, paint particles can be washed off the stones and into the soil, which nourishes vegetables and fruits. The chemicals will get into the food. Plastic with chemical catalysts or cobblestones with a built-in mechanism are suitable.
Pond decor
An excellent option for decorating an artificial pond. Plastic stones with chemical catalysts are well suited for this. They will gain light during the day, naturally, and release it at night. The product will not lose its appearance in water and will not deteriorate. But the other two options may suffer from water.
Painting tiles and borders.
It is common to apply luminous compounds to paving slabs and other elements.
- It marks the borders of the edge of the flower bed.
- Apply to the path. It is covered in whole or in part, highlighting some areas.
- Decorate hedges.
- Decorative elements of the fountain.
These and other options will help you navigate and decorate the area with glow-in-the-dark stones.
How to wear
Fluorite can be worn in the form of jewelry, key chains, or you can put the raw mineral in your pocket or sew it into your clothes. In order for the stone to remain intact, it must not come into contact with solid objects.
It is believed that the magical properties of fluorite are more pronounced if you wear earrings, rings or rings (preferably on the ring finger). The frame can be gold or silver.
Pendants, necklaces, and beads look beautiful, but they need to be worn infrequently and not for long, otherwise the stone can have a depressing effect on the owner’s psyche and lead him astray. The abundance of stone (for example, in bracelets) will have the same effect on the owner.
Fluorite is suitable for people of any age.
For men, rings, tie clips, and cufflinks are made from it.
The rich color palette of the stone, moderate or strong shine allows women to choose jewelry for any outfit.
Price
Fluorite is an inexpensive mineral, so anyone can buy it. Examples of prices in Russian online stores:
- bead (8 mm) - 20 rub. ;
- tumbling (2 cm) - 160 rub. ;
- earrings (brass) - 230 rub. ;
- cabochon (26 x 22 x 11 mm) - 270 rub. ;
- crystal (8 cm) - 708 rub. ;
- intergrowth of crystals - 840 rub. ;
- necklace— RUB 1,163. ;
- rosary (30 grains, d=10 mm) - 1,220 rub. ;
- ball (4.5 cm) - RUB 2,316. ;
- figurine “Owl” (15 cm) — RUB 13,000.