By learning how to do gilding at home, which is not as difficult as it might seem at first glance, you can give a second life to your favorite copper and silver jewelry. Products made from gold have been very popular among both women and men for many years. To own such products without significant costs for their acquisition, it is enough to master the technology of gilding.
Both non-ferrous alloys, steel or cast iron can be plated with gold
What is gilding and gilding. History of the art of gilding - a hot method from antiquity
Gilding is a thin layer of gold that is used to cover a variety of surfaces to give an item the appearance of a precious metal. Thickness varies from 1 to 25 microns.
The technology allows the use of gilding in jewelry design, interior design, architecture, cosmetology and the food industry.
The art of gilding has been known for many millennia. Previously, two methods were used: cold, hot. The cold method was based on attaching gold plates to the surface. The hot method involves the use of fire: gold and mercury were applied to objects. The surface was burned, the mercury evaporated, and the gold stuck tightly to the base. Knowledge of the toxic effect and severe poisoning of workers at work forced the abandonment of the method.
The first gold-plated jewelry and household items were discovered back in antiquity. The ancient Egyptians were among the first to gild the sarcophagi of the pharaohs. Then China and Ancient Rus' mastered gilding. Since then, there have been many techniques for coating various objects with gold.
Leaf gilding technique
The galvanic gilding technique has been known since the 19th century and is considered safe for use by jewelers. Electroplating was a new stage of development in the era of gilding.
Active study of the properties of gold made it possible to use gilding methods in painting, icon painting, forging or casting for furniture decoration, and the architecture of buildings from the design of ancient Egyptian tombs to the pompous Baroque style of Bartolomeo Rastrelli.
Ready solutions for electroplating
Gilding the token
You can have your items professionally updated with gilding at.
The company conducts research in the field of electroplating, developing new technologies in this direction.
The goal of the scientific work is to deposit metals on various conductive and non-conductive substrates. Our specialists will help you apply gilding, palladizing, copper plating, nickel plating or silver plating.
The company's craftsmen accept orders of any complexity and work according to customer requirements. Test samples are made to determine the order price for a large volume of work.
Another area of activity is the professional production of electrolytes for enterprises and individuals. You can order any solution, even according to your own recipe - it will not only be manufactured, but also tested. The company also provides training for technologists and craftsmen.
You might also be interested in:
Types of gilding
Gold plating electrolytes
Gilding
Electroplating training
Gordienko Anastasia Vadimovna Author of the materials Position: chief technologist of 6 Micron LLC Education: higher Experience in galvanizing: 13 years
When placing an order online, get a 10% discount!
Our priority is an individual approach to each order and the quality of the work performed!
Send a request or ask a question:
4.9/5 — (422 votes)
What metals can be gilded
Jewelers quite often use gold plating for silver items. Bronze, copper, cast iron, brass, aluminum, galvanized iron and other well-known metals can also be coated with gold. Certain industries practice gilding of wooden, clay, plastic, and plaster objects. A gold layer can be added to ceramics and cardboard.
Gilding decoration
Why is copper gilding necessary?
Gilding of copper, performed by our company’s specialists, instantly increases the value of a base metal product, putting it on a par with jewelry. The thinnest gold layer applied to a copper “trinket” turns it into an expensive product made of noble metal. The advantages of gold-plated copper jewelry are as follows:
- the ability to use semi-precious or precious stones in decoration;
- coating copper with gold extends the service life of the product;
- low cost.
Also, an undeniable advantage of gilding copper in our company is the acquisition of anti-corrosion properties by your favorite product, which significantly extends its service life.
Gold color technologies
The gilding technique changed, improved and was divided into two main methods of application: clay and Mordan glue.
- Antique clay gilding: multi-layer surface preparation using a complex primer, polyment for the application of gold leaf itself for relief. This method is suitable for processing wooden coverings indoors, clay-based products, and icons. Today there are special mixtures, ready for use, which are easy to buy in St. Petersburg, Moscow and other regions of the country.
- Popular gilding on glue: the gold layer is applied to a special oil varnish-mordan. Advantages of the method: simplicity, accessibility, moisture resistance. This technique is used by craftsmen more often and on various surfaces: metal, wood, plaster, mastic, stone.
Home gilding practice
The method of gilding at home, rather than in a specialized laboratory, is quite complex and not always feasible. The chemicals needed for gilding at home are not always available to hobbyists. The gilding process itself is not at all simple; it requires skills and experience.
The mechanical technique is more often called gilding. Gilding involves covering items with gold foil. Thin sheets of soft gold are applied to the product; the thickness of the sheets is determined to be several microns.
Mechanical gilding method
In the mechanical method, two main approaches can be distinguished:
- Oil direction. This direction is based on the technology of gluing gold leaf onto oil varnish. The advantage of this method is the ability to gild products made from a wide variety of materials, including metal objects and plastic parts. Among the disadvantages, noteworthy is the effect of moisture on the gold-plated surface. An object made in this way has a matte surface, without a characteristic shine.
- The technology of adhesive gilding has undergone virtually no changes since ancient times. Up to 10 layers of polymer are applied to a specially prepared surface, then gold leaf is applied to the surface.
The ideal basis for this gilding procedure is wood and polyurethane. The gloss obtained as a result of this method can satisfy the most demanding consumer requirements. It is possible to carry out gilding in this way only in a perfectly dry production room.
Electrochemical method of applying gold plating
Electrochemical gilding also has several varieties:
- When gilding products using the galvanic gilding method, the metal part is immersed in a composition of noble metal salts. When an electric current is passed through the mixture, particles of gold salts with a positive charge are concentrated on the original object. As a result, the output is a strong layer of gold, the chemical strength of which is quite high.
- The most effective method is the electrochemical metallization method. The use of gels containing metal and a specialized power source makes the gilding process faster and more technologically advanced.
The coating applied in this way has a molecular bond with the original surface, and its durability increases significantly due to the presence of colbate salts. Technological advantages include the absence of the need for a container for gilding, and gold plating can be done on objects of various sizes. There is no need to disassemble the original product; gold plating settles only on conductive surfaces.
At home, the method of rubbing metal is often used. To do this, gold chloride is placed in a solution of potassium cyanide, adding chalk, bringing the solution to a slurry state.
If gold chloride is dissolved in ether, then gilding can also be carried out with this solution. After the ether evaporates, a thin layer of gold with a characteristic shine is formed on the surface of the original part. Using this solution, you can use a pen to make golden designs on metal.
Types of gilding by a specialist
Several simple, accessible gilding methods are actively used. Gold leaf or gold leaf can be used to cover interior elements, household items, and jewelry.
Leaf tinsel for coating
The leaf method involves the use of 960 gold sheet. The method is used to cover temple domes, icons, sculptures, fountains, clocks and other parts of decor. Recently, the use of edible gold has become popular for decorating cakes, desserts, sweets, and even meat dishes.
For such purposes, tinsel is used with a plate thickness 10 times thinner than the cross-section of a human hair.
Gold leaf
Inexpensive use of gold leaf
Potal is today considered a cheaper alternative to gold plates. Due to the absence of precious metals, a gram of such coating costs tens of times less. Produced in the form of gold foil or strips. It is a copper-aluminum or zinc alloy.
By changing the composition of the alloy, you can imitate gold, silver, bronze material. A design can be applied to such surfaces.
Rhodium can protect jewelry from damage.
DIY gilding
Gilding at home involves several methods:
- processing items using gold chloride;
- zinc contact;
- processing by galvanic methods.
These methods can be used at home, but will require chemicals, tools and special equipment, which are not available to everyone.
Gold plating
Gold Chlorine Staining Recipe
For gold plating at home, gold chloride is used, which can be made by melting the precious metal in a “hellish mixture” consisting of a 1:3 ratio of nitric and hydrochloric acids.
Electrolytes for gilding metal surfaces.
2.1 Cyanide electrolytes.
When gilding, cyanide and non-cyanide electrolytes are used. Cyanide compounds, in turn, are divided into acidic, alkaline and neutral. All cyanide electrolytes contain some amount of free potassium cyanide.
In alkaline and neutral solutions, gold is found mainly in the form of the K[Au(CN)2] complex. The presence of the trivalent complex K[Au(CN)4] is possible in small quantities.
2.1.1 Cathode process in cyanide gold plating electrolytes.
The reaction that determines the electrode potential:
[Au(CN)2]- = AuCN + (CN)- AuCN = Au+ + (CN)-
Cathode polarization curves for an alkaline electrolyte are shown in Figure 1; the polarization of gold electrodeposition from acidic and neutral solutions is in the region of more positive values.
Figure 1 - Polarization curves of gold in alkaline cyanide electrolyte (g/l Ag in terms of metal): 1 - 17, 2 - 30.
Let's look at the polarization curves in Figure 1 in more detail:
• In sections 1 and 2 of the curves at a potential from -0.4 to -0.6 V, AuCN or Au2(CN)2 participate in the elementary reaction. • In the 3rd and 4th sections at a potential from -0.7 to -0.9 V - [Au(CN)2]-. • In section 5 - [Au(CN)2]- and H+. Under the influence of a strong electric field in the DES at the cathode, the adsorption of the [Au(CN)2]- anion occurs with the positive end towards the cathode, which facilitates the discharge process:
[Au(CN)2]- + e = [Au(CN)2]2- [Au(CN)2]2- = Au + 2(CN)-
The trivalent gold complex has been studied very little. One of the researchers was A. Knodler. It is known that heating an alkaline electrolyte and increasing the pH promotes the formation of monovalent gold. If we consider the reactions:
[Au(CN)4]- = [Au(CN)2]- + (CN)2 (CN)2 + 2(OH)- = (CN)- + (CNO)- + H2O [Au(CN)4 ]- + 2(OH)- = [Au(CN)2]- + (CN)- + (CNO)- + H2O
then it becomes obvious that the decomposition of the trivalent gold complex should indeed accelerate with increasing pH (increasing OH- concentration).
In neutral and acidic solutions, the trivalent complex is stable even when heated.
If a trivalent gold complex is present in the solution, its decomposition is facilitated by metallic gold and free cyanide:
[Au(CN)4]- + 2Au + 2(CN)- = 3 [Au(CN)2]-
Metallic gold can be anodes, gold-plated parts left without current, particles of gold sludge and coating residues that have fallen into the bath. The cathodic current efficiency in alkaline solutions can reach 70-80%, neutral - almost 100%, acidic - 30-40%. Operation at low current densities is accompanied by a decrease in VT. The already deposited gold coating dissolves according to the following reactions:
2Au + 4KCN + O2 + 2H2O = 2K[Au(CN)2] + H2O2 + 2KOH 2Au + 4KCN + H2O2 = 2K[Au(CN)2] + 2KOH
2.1.2 Anodic process in cyanide gold plating electrolytes.
The anodic process during gold plating has some anomalies - both in the mechanism and in the current output. The anomalies are mainly associated with the possibility of the formation of trivalent gold complexes.
It is possible to use soluble and insoluble anodes:
- Soluble anodes for gilding can only be used in alkaline cyanide solutions. They can dissolve chemically in cyanide, which increases the anodic current efficiency. Over time, the trivalent complex is restored and the anodic process stabilizes.
- Platinized/irridified titanium plates or meshes are used as insoluble anodes.
Popular uses of gold plating
Gilding has been used by people for many centuries in various fields. Its most popular use is in modern technologies of jewelry production and the food industry.
Brilliant gilding
High-quality gilding of modern jewelry
Well-known jewelry brands use gilding in their jewelry, such as:
- SOKOLOV. Most of the products are made from silver. High-grade gold is used to cover them. The layer thickness can reach 5 microns. Palladium serves as a “layer” between the layers. This coating not only improves the appearance of the jewelry, but also enhances its durability.
- Pandora - produces jewelry from precious metals. In his technologies he can use gold plating to cover a special line of jewelry. Pandora Shine is a line of silver products using electroplated coating. This method allows you to give a golden hue and smooth the surface. The Pandora Rose series is distinguished by an interesting pinkish tone of the products. The jewelry is made from a mixture of silver, copper, palladium and 14k rose gold. The pink layer protects the surface from tarnishing and enhances strength.
Turkish gold offered to tourists is also considered popular. Turkish craftsmen use alloys of gold, copper and silver. Gilding is used to improve the appearance. Regardless of the amount of precious metals, jewelry is considered jewelry, with an appropriate value.
Susal in the food industry
Back in the Middle Ages, it became popular to use gold as a food additive; it was credited with medicinal properties. This trend has been revived these days. Gold leaf can be found in pastry shops and restaurants. They are used to cover desserts, cakes, and sweets. Some gourmets prefer gilding with seafood and meat. For convenience, the food industry uses gold in sheets, powder, flakes and granules.
This decoration is considered an important accent of the dish, emphasizing the festive atmosphere.
Food grade gold leaf
What is gold? Properties of gold.
Gold (Au from Latin Aurum
) belongs to group 11 of D.I. Mendeleev’s periodic table. It belongs to typical noble metals and has an uncharacteristic yellow color. The atomic mass and density of gold are high: 196.97 g/mol and 19.32 g/cm³, respectively, and the standard electrode potentials are very positive: Au+ = +1.83 V, Au3+ = +1.52 V.
Gold is distinguished by a high melting point of 1064.18 ° C, pronounced ductility and excellent thermal and electrical conductivity. Its electrical resistivity in its pure form is 0.024 Ohm*mm.
From the point of view of chemical properties, under normal conditions, gold is inert to most acids and does not form oxides.
Gold dissolves only in aqua regia, mercury, alkaline cyanide solutions, concentrated selenic acid at 200° C, perchloric and bromine water, and perchloric acid.
Coatings with gold and its alloys are widely used in electronics and artistic processing of products. Gold can be used as an anti-corrosion coating only if it is non-porous. Its electrode potential is so positive that when paired with electronegative metals such as iron, a very harsh, corrosive galvanic couple will be formed in the pores of the coating.
Designation of galvanic gilding - Zl.
The thickness of the applied coating can vary from 0.025 microns to 20 microns, depending on the purpose.
In terms of its physical and chemical properties, galvanic gold may differ from metallurgical gold. Thus, during the electrolysis process, impurities can be included in gold. This will cause both changes in electrical conductivity and plasticity. Thus, stresses in the coating can be both positive and negative. Electrical conductivity will always decrease. Alloying gold coatings with nickel, cobalt, silver and antimony increases their microhardness, wear resistance, corrosion resistance and fine crystallinity.
Pure gold
the coating has:
- microhardness: 1046 MPa;
- wear resistance: 1 (conditional);
- electrical resistivity, according to various sources: 0.030-0.050 Ohm*m;
- transition electrical resistance at P = 0.2N and current 50A: 0.0031-0.0041. When alloying with nickel
you can get: - microhardness up to 2500 MPa;
- wear resistance 10 (relative to pure gold);
- transition electrical resistance at P = 0.2N and current 50A: 0.0031-0.0041;
- Specific and transition electrical resistance:
| Mass fraction of nickel in the alloy with gold | Electrical resistivity | Transition electrical resistance at P=0.2 and current 50A |
| 5 | 0,150 | 0,0041 |
| 10 | 0,220 | 0,0017 |
| 15 | 0,400 | 0,0100 |
With the introduction of cobalt
:
- microhardness: up to 2990 MPa;
- wear resistance: up to 15 (relative to pure gold);
- Specific and transition electrical resistance:
| Mass fraction of cobalt in an alloy with gold | Electrical resistivity, Ohm*m | Transition electrical resistance at P=0.2 and current 50 mA | Transition electrical resistance at P=0.2 and current 50A |
| 1 | 0,040 | — | — |
| 5 | 0,140 | 0,0062 | 0,0042 |
| 10,1 | 1,150 | 0,0130 | 0,0070 |
| 17,) | 1,150 | 0,0130 | 0,0076 |
When introducing silver
: • microhardness and wear resistance:
| Mass fraction of silver in an alloy with gold | Microhardness, MPa | Wear resistance (relative to pure gold) |
| 4,8 | 1400 | 4 |
| 10 | 1650 | — |
| 16 | 1840 | — |
| 31 | 1850 | 9 |
• specific and transition electrical resistance:
| Mass fraction of silver in an alloy with gold | Electrical resistivity, Ohm*m | Transition electrical resistance at P=0.2 and current 50A | Transition electrical resistance at P=0.5 and current 50A |
| 4,8 | 0,09 | 0,0039 | 0,0022 |
| 10 | 0,08 | 0,0033 | 0,0022 |
| 16 | 0,12 | 0,0033 | — |
| 31 | 0,125 | 0,0040 | 0,0028 |
When introducing antimony
:
• microhardness:
| Mass fraction of antimony in an alloy with gold | Microhardness, MPa |
| 2 | 2030 |
| 5 | 2100 |
| 10 | 2350 |
| 15 | 2600 |
• wear resistance 15 (relative to pure gold). • specific and transition electrical resistance:
| Mass fraction of antimony in an alloy with gold | Electrical resistivity, Ohm*m |
| 2 | 0,088 |
| 5 | — |
| 10 | 0,380 |
| 15 | 0,520 |
• transition electrical resistance at P = 0.5N and current 50A: 0.0050. Alloyed gold alloys have characteristic colors that are often used in artistic decoration. For example, the most popular: green gold is an alloy with silver, rose gold is an alloy with copper.
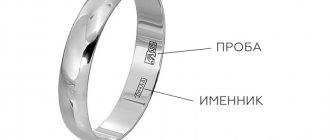
Gold plating markings abroad:
- Vermeil – 333-999 sample (8-24k);
- KHGE - 585 or 750 sample (14 or 18k);
- KGE - 750 (18k);
- Clad in Gold - 750 (18k);
- EP - 750 (18k);
- GEP - 750 (18k);
- Cold Layered - 750 (18k);
- COLD PLTED -750 (18k);
- KARATCLAD - 750 (18k);
- KGP - 750 (18k);
- Layered - 750 (18k);
- Overlay - 750 (18k);
- Technibond - 750 (18k);
- WGP - 750 (18k);
- YGP - 750 (18k);
- Plaque - 750 (18k);
- Gold Clad - 750-999 (18-24k);
- KGB, KTGP - 999 (24k).
Ingots 999.9 fine
Pros and cons of gilding
For many centuries, gold items were an indicator of wealth, power, and status of the nobility. Gold plating was applied to household items and dishes. The material was used to decorate furniture and buildings outside and inside. Based on this, the advantages are:
- reasonable price of the product;
- dishes and other products look as luxurious as possible;
- the golden layer can be restored.
Disadvantages of gilding:
- the coating is short-lived;
- the gilding gradually wears off;
- The service life of the coated item is influenced by the layer and gold plating sample.
Edible gold leaf has a simple composition: 96% gold and 4% silver
What metals and alloys can be gilded?
Gilding of metal surfaces is used in various fields, but most often:
- in industry to create technical coatings that are resistant to chemical attack;
- in jewelry, when decorating surfaces made of ferrous and non-ferrous metals, stainless steel.
Electroplating is used to create costume jewelry. To make gold-plated items, items made from cheap metals are coated with a thin layer of gold. Such “luxury items” are sold at an affordable price, but look like real gold. Electroplating technology is used for bracelets, chains, necklaces, and glasses. The products look elegant and respectable.
No less common is galvanic processing of more valuable metals, for example, gilding of silver or platinum. Jewelry coated with a special composition does not lead to allergies or skin irritation. But such items can often cost at the same level as high-grade silver items.
To impart the required properties to individual areas of surfaces, objects or jewelry can be partially subjected to galvanic gilding. In jewelry, this is how unique things are created.
Any item can be subjected to galvanic gilding, thereby increasing its properties. For example, gold leaf is sprayed onto the domes of churches and used for other decorative purposes.
TOP - 6 tips on how to distinguish fake from gold
Approximately half of all gold products are high-quality imitation, which can be purchased at the cost of the precious metal even from trusted dealers. This means it is important to know how to check an item for authenticity before purchasing. The following are considered signs of counterfeit:
- Uneven layer of gilding. Most often, the material does not fit well or is partially erased during transportation.
- Magnet check. Real precious metal is not attracted to a magnet. But the method is not entirely effective, since the magnetic field is ignored by most metals.
- Defects in the stamp area. When making a fake, the mark from the original is transferred to the copy. Having carefully examined this area, you can find defects, which will be a sign of gilding.
- Chemical exposure to reagents. If you frequently buy gold from different sellers, you should buy a set of special reagents that can be used to easily identify a copy.
- Alcohol helps. After rubbing with alcohol, the gold-plated item will darken.
- Vandal way. By making a small scratch on the surface, you can easily identify a fake. The gilding will be showered with the smallest particles, the damage will stand out.
It is important to remember that the only reliable method of verification is to have the product inspected by a specialist. But you can use it only after purchase.
Prospecting Magnet for Gold Testing
Gilding and silvering technologies
Gilding
Gold has always been the most expensive and rarest metal, and the temptation to pass off less valuable metals as gold has been around for a long time. Since ancient times, silver has been coated with a thin layer of gold, sometimes to imitate gold, sometimes to give the silver additional protection against corrosion. For example, cutlery that came into contact with salt and vinegar was often double gilded, that is, a second layer of gold was applied. Partial gilding was often used to additionally protect the internal surfaces of salt shakers, goblets, communion bowls, etc., as well as to create a contrasting decor.
Since the difference in the value of plain and gilded silver has always existed, jewelers, scribes, clerks, etc., in all types of accounting, indicated whether the item was made of plain silver or gilded, and what type of gilding - partial or double.
Before the invention of galvanic (electrolytic) gilding, the method of mercury or fire gilding was widely used (in Ancient Rus' it was called “burnt gold”). This method was also used to gild bronze, and it was also used to cover engravings on silver with gold. Since mercury gilding would wear off over time when items were cleaned, old items were often re-gilded using the electrolytic method.
Mercury gilding is a very dangerous process, since mercury vapor is extremely harmful to health. This method is now banned in most countries.
Gilding of metals usually involved a process very similar to the old Sheffield method of silvering (see below). Gold foil of a suitable standard (usually 9 karat) was soldered to a base metal base, which was then rolled to the desired thickness. The base was usually bronze or an alloy of copper with zinc and nickel, known as nickel silver or cupronickel.
The finest gold foil, or gold leaf, has been used to decorate both metal and non-metal surfaces, such as wood, since ancient times - for at least 4,000 years. To do this, thin sheets of gold leaf were placed on a specially treated and prepared surface and rolled so that they adhered tightly to it, displacing all the air. If necessary, not one, but several layers of gold were applied. This is a cold process, and for its high-quality execution, guaranteeing long-term preservation of the coating, a qualified specialist was needed.
Preparing sheets of gold leaf is an extremely labor-intensive process. First, metal of 23 Y4 carat purity (i.e., almost pure gold) was rolled to produce strips 1 Y4 inches (32 mm) wide and 1/1000 inch (0.025 mm) thick. The strips were cut into squares and laid over with square sheets of special thin tracing paper measuring 4x4 inches (100x100 mm). A stack of these sheets of foil lined with tracing paper was tied together with parchment and placed on a wooden block, and then hammered for half an hour with a heavy 9 kg hammer. Transferring the gold foil with tracing paper is necessary to prevent the sheets of gold leaf from sticking together and sticking to the working surface of the hammer. In Rus', in the old days, film taken from the entrails of animals was used to lay sheets of gold leaf during flattening.
Gradually the gold became thinner, approaching the edges of the tracing paper squares. Then the resulting sheets of gold foil were cut into four parts, transferred again with tracing paper, combined into a pile of 800 leaves and crushed further until the gold again reached the edge of the spacer sheets. After this, the thinnest sheets of gold foil were separated with wooden tweezers, again cut into four parts, wrapped in parchment and beaten for another five hours. As a result, the sheet of gold leaf became 250 times thinner than the original strip. After beating was complete, the gold leaf was cut into 34 inch (83 mm) squares and stacked into stacks of rough, sulfur-free porous paper. These sheets were used by the gilder in his work.
French silver plating (plaque) and applied silver
Just like with leaf gilding, when silvering according to the method of the old French masters, a silver sheet
foil heated to a high temperature was applied to the cold surface of the base metal and polished while hot for a more durable connection of metals. Often the base was copper, and workshops working with Sheffield silver plated copper sheets (see below) used French silver plating to correct defects in their pieces, such as flaws in the silver plating at corners and raised areas - traditionally problem areas for this technique. By adding silver foil layer by layer to these areas, it was possible to achieve any desired coating thickness.
The French method of applying applied silver did not provide great strength of the connection, but was very practical for minor repairs - after all, the silver plating on many products, primarily made using the same method, partially came off over time. It was also not suitable for subsequent engraving of products - the foil layer was separated from the base when cutting through it with an engraving tool.
Another technology was used to coat steel parts of various products with silver, such as the blades and handles of table knives, spatulas, tongs, etc. It was invented a long time ago by craftsmen who made cutlery in Sheffield, England. Starting from the 15th century, gunsmiths began to use it for silvering parts of bladed weapons, spurs and other equipment. This method could be used to silver almost any metal, provided that its surface was thoroughly cleaned and leveled beforehand.
Technologically, the method consisted in dipping the cleaned object first into ammonia and then into molten tin. Silver foil of a high purity, at least sterling standard (this standard was established back in 1327 and then enshrined in 1625 by the guild regulations of the Sheffield guild of cutlery craftsmen), was placed on the item and pressed tightly so that there was no air between the foil and the layer of tin came out completely. Then the foil was processed with a heated soldering iron, the tin melted, and the silver was combined with the base metal.
The main disadvantage of this coating is that with strong heating, the silver can peel off. In addition, moisture could penetrate through the coating and cause corrosion of the base metal, after which the silver layer would swell. In this case, it was no longer possible to repair the product.
Sheffield silvering
The Sheffield silvering method was invented by master silversmith Thomas Boulsover around 1742. It consisted of fusing a silver sheet onto a copper ingot in a furnace and rolling the resulting silver-coated ingot into sheets that served as blanks for the production of a variety of products. This method had significant advantages over all previous silvering methods, and soon other Sheffield cutlers mastered the Boulsover method. A short time later, a whole industry branch emerged in this city, producing mainly cutlery and candlesticks, which were eagerly purchased by representatives of both the middle and upper classes.
The method of fusing silver sheet to a base was soon used in other cities, primarily in Birmingham, where a large specialized enterprise arose in 1760. There are known workshops in Sweden, France and Russia that also practiced this method of silvering.
After the electroplating method of silvering was patented in Britain in 1840, the Sheffield method fell out of use almost everywhere, and by the end of the 19th century it was used only in a few workshops for the production of buttons, beer mugs and other products where special coating strength was required under intensive conditions. consumption.
In the Sheffield method of silvering, a sheet of pure silver was applied to the copper ingot after thorough cleaning. The standard bar was 9 inches (23 cm) long, 21/2 inches (6.5 cm) wide and 1 Y? inches (4 cm) thick. The amount of silver taken could vary depending on the intentions of the craftsman, but most often, if measured by the thickness of the metal, 1/8 inch (about 3 mm) of silver was taken per 11/2 inches (about 40 mm) of the ingot. A heavy iron pig was then placed on top of the silver sheet and the stacked pieces were hammered through it to force out all the air between the silver and copper and ensure close contact between the two metals.
After beating, a copper sheet of approximately the same thickness (1/8 inch, or 3 mm) was laid over the silver sheet to protect it in the kiln. The surface of the copper sheet in contact with the silver was first coated with a solution of lime to prevent the sheets from fusing, then all three metal plates were tightly bound with iron wire. The silver plate was covered at the ends with a layer of crushed borax, which acted as a flux, melting at a low temperature and preventing air from penetrating to the junction of the metals, otherwise it could oxidize the metals and prevent them from joining.
A metal block of three plates was placed in the furnace and placed directly on top of the fuel, usually coke. When heated to a certain temperature, silver and copper fused, forming a single ingot. This was due to the fact that an alloy easily formed at the point of contact between the metals. The melting point of this alloy was lower than that of pure silver or copper, and this made it possible to fuse two plates without raising the temperature to the point where the plates would completely melt.
The ingot was then removed from the furnace, the binding wire was cut off, the protective top sheet of copper was removed, and the ingot was cleaned. Around 1760, Sheffield craftsmen also developed the technology of applying a silver coating to both sides of a copper plate.
The silver-plated ingot was rolled into a sheet using powerful mechanical rollers. Because copper and silver behave similarly when machined, they were rolled out as a homogeneous ingot of metal.
Many items were made from silver-plated Sheffield sheet using the technique of punching and chasing. Another processing technique that allowed for mass production to a certain extent was stamping. Although dies were expensive to produce, they could be used to make a variety of blanks and parts that were used in different combinations to produce a variety of products. In 1820, Sheffield silver-plated sheets began to be processed using the rolling technique, which made it possible to expand the range of products obtained and reduce the cost of some types of products. Parts of Sheffield sheet products were joined by soldering, usually with soft lead-tin solder, but sometimes brass and silver hard solder was also used. Tall vessels were made by bending a metal sheet around a mold and sealing the seam. These seams were often sought to be hidden by sanding, but if they can be found on a piece they can help identify the piece as being made from Sheffield sheet. With galvanic silvering, the seams on the products are not visible.
One of the main problems with using Sheffield sheet was that the copper base was visible at the ends. They tried to fight this in different ways. One of the earliest was to bend the edges of the product inward. Later they began to solder a silver strip or wire to the edges of the sheet. In 1768, a method was invented and improved in 1780 to coat copper wire with silver, which was used to decorate the edges of a sheet in a product. Around 1790, decorative trim pieces began to be stamped from thin sheet silver and soft soldered onto the product. In this case, in particular, they began to use decorative overlays, which were soldered along the edge of the product, and the protruding part of the nozzles could be bent to hide the copper at the end of the sheet.
In 1770-1780, openwork products made by cutting with a jigsaw came into fashion. However, this method, popular among silversmiths, was not suitable for Sheffield sheet, since the holes made with a jigsaw revealed copper in the middle of the sheet. Therefore, in Sheffield, for the production of openwork products, they began to use a press, with the help of which a hole was made at the intended point with a steel punch. Due to the plasticity of the metal, the silver layer was deformed, stretched and hid the copper.
Sheffield sheets were also decorated with engraving. This was not easy, as the engraving could expose the copper. Therefore, thin plates of silver or Sheffield sheets with a thicker layer of applied silver were often soldered onto the product in places intended for engraving. Beginning around 1780, Sheffield craftsmen began to cut holes in their products in the places where engraving was intended, and solder sheets of thicker silver into these holes. The solder seam was usually hidden by engraving, but it can be seen from the inside.
In 1810, instead of soldered plates, they began to use special overlay shields for engraving, which were made from thin plates of pure silver. They were placed on the surface of the product, heated and soldered to the silver coating, after which they were sanded flush with the surface. Because the shield was made of pure silver and the Sheffield plate was covered with sterling silver of a different purity, they tarnished differently over time and the presence of the shield became noticeable. The presence of such shields is one of the characteristic features of products with Sheffield silver.
Items made from Sheffield sheet required finishing. If a single-sided silver-plated sheet was used, the other side was covered with tin, which was melted and then poured over the product to distribute it evenly. This hid the copper and prevented it from coming into contact with food.
The final stages of finishing were cleaning, sanding with steel or agate floats and polishing.
Galvanic silver plating
Electroplating began to be used in Birmingham, where in 1840 brothers George and Henry Elkington patented the process of electrolytic deposition of the metal. It is believed that they learned about this phenomenon from a certain John Wright, who discovered it when, in 1833, he repeated the experiments of Michael Faraday and tested the laws of electrolysis he had derived.
It should be noted that already in 1800, Alessandro Volta's electric battery (the so-called Voltaic column) made it possible to obtain a metal coating, and 14 years later, jewelers announced that they had succeeded in producing a “plated cup” using the electrolytic gilding method. But the Elkington brothers' patents, filed in 1836–1837, were the first attempt to register the discovery, and in 1843 the brothers were already issuing licenses to manufacture the process.
Silver was the first metal used for industrial electroplating, and the nature of the process has changed little over the past century and a half. A bath was prepared with a solution of potassium silver cyanide (sodium cyanide can also be used) and a small amount of potassium cyanide. An anode made of almost pure 100% silver was used for the work (if the purity of the metal was less than 99.97%, it was no longer suitable for the process), and the product, carefully prepared and cleaned, was lowered into a bath suspended on copper wires. A weak current was passed through the solution. The bath was at room temperature, the current voltage was controlled by a voltmeter, which should be in the range of 1-1.5 volts, due to which the anode remained clean.
For the process to be successful, the product had to be absolutely clean, without the slightest trace of dirt, grease or oxides. After cleaning it could not be touched. After keeping the product in the bath for 15 seconds, it was checked to see if the coating was well fixed. The process then continued, and when a satisfactory result was achieved, the item was washed and cleaned.
Almost any metal can be silvered in this way, including iron, brass and copper. Nowadays, nickel silver or cupronickel is most often used for silvering.
During deposition, the worker slightly rocked the product to ensure even deposition. Using a pure silver anode, it was possible to obtain a coating of any thickness. The side of the product that was closer to the anode was coated with a thicker layer, and the inner walls of the vessels received a very thin coating, unless special measures were taken. The difference in coating thickness on the same product could reach 6 times. An especially thick coating was applied to the undersides of spoons and bowls, which were subject to the most wear and tear. To obtain a good coating, approximately the thickness of tissue paper, the product had to be kept in the bath for approximately two hours.
As the silver was coated, the product became white, like porcelain, but if a special electrolyte containing carbon bisulfate was used, a shiny surface was obtained, which made final polishing easier. The product was washed, dried and polished - first with cotton swabs with sanding paste, then with crocus (red polishing powder) on felt swabs and, finally, with the same crocus manually.
Nowadays, gilding, as a rule, is also applied electrolytically. The process is very similar to that described above, and differs only in the bath temperature (60-65 degrees) and current voltage ('/t - 2 volts). Since potassium aureate salts and the gold anode (if one is used) are expensive, fairly thin layers of gold are usually deposited.
High-quality gilding is done by successively applying many layers, and in between, the product is processed with a stiff brush. A matte surface can be obtained by sandblasting the product before gilding. For partial gilding, for example, the inner walls of vessels, salt shakers, etc., as well as for decorative partial gilding, areas that are not planned to be gilded are covered with a layer of some insulating substance, for example, shellac.
Sometimes a strong, white, shiny platinum group metal, rhodium, is used to coat silver and jewelry, as well as base metals and alloys. But such a coating is expensive, and it looks rough on silver. On the other hand, it is practically not subject to corrosion.
Magazine "Antiques, art and collectibles", No. 72 (December 2009), p.54
Rules for caring for gold plating: wearing, storing, cleaning
Of course, the gold plated layer will not last forever, but you can extend its life by properly caring for and cleaning the surface. It is important to follow the following rules:
- gold-plated items should be stored separately from others;
- It is better to remove jewelry before the pool, shower and gym: limit contact with humidity and water;
- to clean jewelry, wipe it with alcohol using cotton pads;
- To prevent gilding, it is enough to regularly wipe with a dry piece of suede cloth;
- It is recommended to show the products to craftsmen once a year and, if necessary, restore the coating.
Section question - answer
Does gilding darken or not?
Expert opinion
Grishanov Mikhail Petrovich
Jeweler, director of the Grishanov and Co. workshop
Over time, gold plating may slightly change color and darken. When worn and stored correctly, gold-plated items retain their color and shine for a long time. Periodic cleaning prevents items from becoming dull, and wiping with alcohol will prevent darkening and loss of shine.
How to coat metals with gold at home?
Expert opinion
Pribrezhny Gennady Valentinovich
Jeweler 6th category
Gilding at home is carried out using chemical reagents. Several methods can be used:
- immersion in a chemical composition with zinc contact;
- use of electric discharge in solution;
- staining with chlorine gold.
Gold jewelry, bracelets
Is it possible to gild metal yourself without gold?
Expert opinion
Grishanov Mikhail Petrovich
Jeweler, director of the Grishanov and Co. workshop
Gilding without using gold at home is possible by using gold leaf. It does not contain noble metal components and is made from an alloy of copper mixed with aluminum or zinc.
Methods and methods of gilding metal
The quality and appearance of the coating depend on the technology used. The most common methods of applying gold are:
- mechanical;
- chemical gilding using active substances;
- galvanic gilding.
For each method, different chemicals and tools are used.
Chemical method
Chemical gilding is the application of gold chloride to the item to be treated. To create a reagent, you must sequentially perform the following steps:
- The metal is broken into small pieces.
- The elements are placed in a pre-made mixture. The reagent is made based on a compound of hydrochloric (30 g) and nitric (10 g) acids.
- For each gram of spraying, about 10 ml of reagent should be consumed. Therefore, it is necessary to prepare the required amount of solution in advance.
Acids in everyday life should be handled carefully, avoiding contact with the skin of the hands and mucous membranes.
The ingredients are mixed in a porcelain flask, and dissolution can take three days. Once the preparation of the noble metal is complete, it is evaporated at 80 °C to form a liquid solution. To obtain a homogeneous composition of the required consistency during the evaporation process, the solution must be periodically shaken with a glass stirrer.
The following is prepared directly for the process:
- filtered water heated to 60 °C;
- golden chlorine salt – 15 g;
- sodium chloride or potassium carbonate – 65 g.
Items are processed after preparation has been completed in advance. To remove grease, it is recommended to wipe with 20% caustic soda, then wash in a 25% soda solution.
The item is placed in the mixture, and after a certain time (according to the type of metal) the surface turns gold-plated. After natural drying, the item is wiped with a soft, dry cloth. The surface is polished with a woolen cloth until it shines.
Mechanical gilding methods
Gilding gives the product certain properties that make it aesthetically attractive and resistant to environmental influences.
Mechanical processing is performed infrequently, because obtaining a uniform surface layer is not easy. This technology has the following features:
- It is necessary to use a special paste, produced in a special laboratory or independently. Paste ingredients may vary significantly for different products.
- The coating applied mechanically in domestic conditions, unlike galvanic coating, has a small thickness. Therefore, such products will not last long.
- The most common solutions are based on cream of tartar and yellow blood salt.
- To form a homogeneous porridge-like composition, rubbed into the surface of the product with a woolen rag, the substance is thoroughly mixed.
- Before applying gold plating, it is necessary to degrease the surface.
- The paste is applied in one layer with an even distribution of the golden mixture. When rubbing, it is necessary to avoid contact of the substance with the skin of your hands.
Mechanical gilding, unlike galvanic gilding, is a complex process, used in everyday conditions only for individual parts of objects. Other methods involve complete immersion of the part to be treated in a special solution.
Galvanic gilding of metals
Gold plating of sufficient thickness and quality is obtained by using a special technology that involves immersing the item in an electrolytic composition. The galvanic method is intended for processing products of almost any size; in fact, it is one of the types of electrochemical reactions.
It is necessary to take into account that the composition of the galvanic solution affects the shade acquired by the surface.
At home, an electrolytic solution for creating galvanic gold plating is prepared in the following sequence:
- 60 g of sodium phosphate is added to filtered water with a volume of 0.7 l;
- to dissolve 2.5 g of gold chloride, add 0.15 liters of water;
- 1 g of potassium cyanide and 10 g of sodium sulfide are dissolved in 150 ml of liquid.
When gilding using the galvanic method, the ingredients used to make the mixture must be handled carefully, avoiding spillage or contact with the skin, eyes, or mucous membranes.
The galvanic method involves heating the solution to 60 °C.
An anode is installed to electrolytically deplete and initiate reactions, reducing operating time and increasing the efficiency of the galvanic method. Gilding items in these solutions using the galvanic method takes about 15 hours, and a matte gold film is formed on the surface.