Chemical and physical properties
Bismuth is a metal that can be compared to water. In a liquid state it is denser than in a solid state. This is also true for bismuth. When melted, it, like ice, decreases in volume. It turns out that solid metal is lighter than fluid metal. Due to compaction during melting, bismuth reacts unusually to pressure.
The temperature at which a substance changes from solid to liquid drops as it rises. A fluid mass can be obtained already at 270 degrees Celsius. At 1000 degrees, bismuth burns . If you apply pressure to other metals, their melting point will only increase.
Bismuth is the main, most powerful diamagnetic in nature. This means that the metal is repelled from both magnet poles. If you place the ingot between the plus and minus, it will stand in the center. The phenomenon is called diamagnetic levitation. The force of bismuth is so strong that it can detach the magnet from its support.
When comparing an element with antimony, the metallic properties differ. In bismuth they predominate. Antimony has more non-metallic parameters, for example, no pronounced shine. The 83rd element of the periodic table sparkles, “giving birth” to pinkish flashes.
Distinguishes between bismuth and plasticity. Metal is soft but at the same time fragile. The oxidation of the element is ambiguous. In dry air, the substance can be mistaken for a noble one - a patina does not form. In a humid atmosphere, bismuth oxide . The surface of the metal becomes covered with a film and becomes cloudy.
instructions for use for the metal , chemists indicate that it does not react with alkalis. The element is also inert to dilute acids. But, if you use concentrates, bismuth salts fall out. The reaction with metals is active, bismuthides are formed. This is a group of minerals, including maydonite, frodite and michenerite.
Answers to user questions
We will answer several basic user questions regarding metal.
Bismuth is a light or heavy metal
Metals with an atomic mass greater than 50 form the group of heavy metals. Bismuth falls into this company.
Is bismuth harmful or not?
One of the unique properties of bismuth is that, being a heavy metal, due to its low solubility in water, it does not have a negative effect on living organisms, unlike its “colleagues” . Bismuth is not toxic and does not accumulate in the body if taken in acceptable quantities (up to 20 mcg).
Poisonous properties appear only in bismuth salts that are easily soluble in water - their action is similar to mercury salts. When interacting with some pharmaceutical drugs, toxic substances can also be formed. Long-term use of bismuth-containing medications is contraindicated in patients with kidney disease.
Bismuth poisoning is rare. Its symptoms:
- gastrointestinal disorders;
- inflammation of the oral mucosa;
- dermatitis;
- cardiac dysfunction;
- loss of appetite and sleep.
The symptoms are unpleasant, but side effects can be avoided if you take the medications under the supervision of a doctor.
Applications of bismuth
Bismuth found application in metallurgy. The element is necessary to create low-melting alloys. Metal is added, for example, to Voodoo. It is used in fire protection systems. The melting index of the alloy is lower than the boiling point of water.
Enterprises producing cast products of complex shapes are also eager to buy metal. It is important to maintain the accuracy of the parameters. The property of bismuth to increase in volume as it hardens is useful The addition of metal helps the alloys adhere closely to the forms and repeat their contour 100%.
By combining with manganese, bismuth acquires ferromagnetic properties. When placed in a magnetic field, the alloys themselves become magnets. Compounds based on bismuth and manganese are used for their production. Oxides of the 83rd element are used in the production of ceramics, glass, and optical devices. Here bismuth plays the role of a catalyst.
Bismuth preparations are on pharmacy shelves. The metal tribromophenolate, as well as the xeroform, is useful in pharmaceuticals. These compounds actively fight bacteria. Therefore, powders with bismuth are used in wound healing, disinfection of burns and fistulas. Metal is added, for example, to Vishnevsky’s ointment. Bismuth nitrate is known to doctors as an astringent and mild laxative. The connection is called vikair.
Tripotassium bismuth is the basis of antiulcer drugs. They are available in the form of thin-coated tablets. Some of them are prescribed for gastritis. Thus, tripotassium bismuth dicitrate contains the drugs De-Nol, Trimo, Ventrisol and Pilocid.
Compounds of the 83rd element are also added to medications for syphilis. Its causative agent is spirochetes. These bacteria die in the presence of bismuth, which binds sulfide groups of microorganisms.
Bismuth has also taken its place in cosmetology. Oxochloride substances - glitter in many decorative products. Powders, eye shadows, and blushes with a radiant effect often contain 83rd metal. Cosmetologists paid attention to it back in the Renaissance.
Then there was a fashion for snow-white skin - a sign of the aristocracy. Bismuth nitrate helped representatives of the upper class “not fall face down in the dirt.”
The salt was called Spanish white and was used as powder. Bismuth nitrate is a salt. Metal salts are needed by road workers. Have you seen signs on the highways, the pictures on which begin to glow if headlights are directed at them? The secret of color flashes is bismuth salts.
Process specifics
It is worth mentioning separately about the extraction of bismuth from crude lead. This process is specific in that it involves the release of metal using calcium or magnesium. In this case, bismuth, having the form of a CaMg2Bi2 compound, accumulates in the upper layers.
How is the metal subsequently purified from magnesium or calcium? By melting it under an alkaline layer with the addition of the oxidizing agent NaNO3. The resulting substance is then subjected to electrolysis to produce sludge (waste substances). This product is melted into crude bismuth.
It is important to note that the hydrometallurgical method for obtaining this element is characterized by higher economic indicators and the corresponding purity of the resulting substance. This method is based on the dissolution of bismuth-containing ores, alloys and intermediates. For this, hydrochloric and nitric acids are used.
Dissolution is followed by leaching of the resulting liquid. To carry out this process, sulfuric acid or sodium chloride solutions are used. This is the last step, then the bismuth is extracted and purified through extraction.
By the way, there are also methods of two-stage distillation, zone smelting and hydrometallurgical refining. They are used to obtain the purest bismuth.
Bismuth mining
Rare metal. It's about bismuth. Instructions for its extraction usually concern the ores of lead, tin, copper and tungsten. They contain about 0.006% 83rd metal. They are extracted as a by-product by leaching the ore. For this purpose, hydrochloric acid is used followed by extraction.
In the production of copper and lead alloys, bismuth is obtained by refining. The metal partially turns into dust and vapor. They are collected and sent for further processing, or rather, restoration. It is achieved electrolytically or by working with lead ingots.
There are also bismuth ores in nature. The content of a valuable element in them is 1%. But such breeds are rare and in small quantities. The total reserve of the metal is estimated at 320,000 tons for the whole world. 240 of them are located in the depths of China. Therefore, the Celestial Empire is the leader in bismuth production. China supplies the market with 6,000 tons per year. Mexico adds 1,000 tons. 100-150 tons are produced in Kazakhstan and Canada.
10,000 tons of bismuth were discovered in Bolivia and Peru. But these countries hardly develop reserves. By the way, element 83, like other metals, is found in native form. The ingots are found in combination with topazes, tourmalines and beryl. The bismuth content in nuggets is about 99%. But such pebbles are even rarer than rare metal ores.
Receiving process
Continuing the topic of what bismuth is, it is worth telling how exactly it is mined.
The production of this metal is based on the processing of ore, as well as lead and copper concentrates, using methods used in the fields of pyro/hydrometallurgy.
There is another method, but it is used only in the case of obtaining bismuth from sulfide compounds. The process involves the processing of copper concentrates, accompanied by precipitation smelting with iron scrap and flux.
As a rule, the process of obtaining bismuth occurs according to the formula: Bi2S3 + 3Fe à 2Bi + 3FeS.
If oxidized ores are used, the metal is reduced with carbon under a layer of flux. This happens at temperatures from 900 to 1000 °C. Carbon, by the way, can be replaced with sodium sulfite. Using this crystal hydrate, it is possible to reduce bismuth oxide at a lower temperature (800 °C).
To obtain sulfide of this metal, soda or sodium hydroxide is used. In these cases, the temperature is set to 950 and 500-600 degrees, respectively.
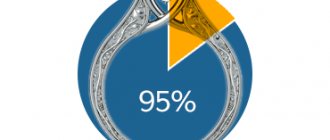
Bismuth price
for bismuth rarely drops below 2,000 rubles per kilogram. This cost is indicated for purchases with minimum volumes. That is, you can save only when ordering from 5, 10, 16 kilograms. If you take only 1,000 grams, you will have to pay at least 3,100 rubles. Standard price - from 4,000 to 6,000 rubles.
Sellers' requests depend on the purity of the metal. Its content in ingots can be, for example, 99%, or maybe 99, 99%. The name of the manufacturer, trader and the country from which the goods were delivered are also taken into account. For Russians, supplies from China are most profitable. If we talk about enterprises within the country, bismuth is sold, for example, by Elektrovek-Stal.
Plant managers set prices based on London Non-Ferrous Metals Exchange indices. As a rule, the cost of a kilogram of bismuth varies between 3,000 – 4,000 rubles. The enterprise's production volumes allow it to trade in centners, which significantly reduces costs for large purchases.
Metal modifications
What is bismuth? Visually, it is a silvery-white metal, shimmering in various shades. Pure bismuth has a predominantly pink cast. A metal in which some other color is dominant is an allotrope.
By the way, there are a lot of them. Modifications occur due to exposure to high pressure. If bismuth is subjected to a temperature of +25 °C and a pressure of 2.57 GPa, the crystal lattice of this substance will undergo a polymorphic transformation. Its shape will cease to be rhombohedral and will become monoclinic.
Also, lattice changes occur at other pressure indicators (5 GPa, 4.31 GPa and 2.72 GPa). And if you bring it to a level of 7.74 GPa, then it will completely acquire a cubic shape. The lattice becomes tetragonal at a pressure of 2.3-5.2 GPa.
The most important connections:
The most characteristic compounds for bismuth are those with oxidation states: +3 and +5. Bismuth(II) oxide, BiO
: Grayish-black crystals. It is obtained by reducing bismuth(III) oxide with metallic bismuth or hydrogen. Oxidizes when heated to 180°C and in humid air. Disproportionates in reactions with acids, e.g. 3BiO + 6HCl = 2BiCl3 + Bi +3H2O.
Bismuth(III) oxide, Bi2O3
: Monoclinic or tetragonal yellow (brown when heated) crystals. Resistant up to 1750°C. Diamagnetic Slightly soluble in water, acetone, liquid ammonia, hydroxides. Dissolves in acids. It is obtained by heating bismuth in oxygen, decomposition of bismuth (III) nitrate, and dehydration of bismuth (III) hydroxide.
Bismuth(III) hydroxide, Bi(OH)3
: White amorphous powder.
Shows basic properties. Slightly soluble in water and concentrated alkalis. Dissolves in glycerin, ammonium chloride and mineral acids. With acids it forms bismuth (III) salts. Bismuth(III) salts
are colorless crystals. substances, soluble salts (nitrate, chloride) to prevent hydrolysis are dissolved in dilute solutions, respectively. acids When dissolved in clean water, they hydrolyze to form precipitates of basic salts (eg Bi(OH)2NO3) or oxo salts (bismuthyl salts, eg BiOCl).
Bismuth(III) iodide, BiI3
: dark brown crystals, insoluble in water, but soluble in alcohols and acetone.
Interacts with solutions of iodides, forming water-soluble complexes: BiI3 + KI = K[BiI4]. Bismuth(III) sulfide, Bi2S3
: Grayish-black rhombohedral diamagnetic crystals.
Has thermoelectric properties. Slightly soluble in water, dilute mineral acids, ammonium sulfide, sulfides and polysulfides of alkali metals. Reduced by hydrogen, carbon, silicon. It interacts with water and is completely hydrolyzed. Bismuth(V) oxide, Bi2O5
: Dark brown powder.
Slightly soluble in water. Dissolves in acids and alkalis. Decomposes when heated. It is obtained by oxidation of bismuth (III) in concentrated alkaline solutions, for example by passing chlorine through a suspension of Bi2O3 in a KOH solution. Bismuth(V) compounds exhibit strong oxidizing properties: H3BiO4 + 5HCl = BiCl3 + Cl2 + 4H2O Bismuthates
are salts of bismuth acids, for example meta- (NaBiO3) or ortho- (Na3BiO4) sodium bismuthate, yellow powder, insoluble in water, strong oxidizing agent: 2Mn(NO3)2 + 5NaBiO3 + 14HNO3 => 2NaMnO4 + 5Bi(NO3)3 + 3NaNO3 + 7H2O
Discovery history:
Bismuth has been known to mankind since ancient times, first mentioned in written sources in 1450 as Wismutton or Bisemutum. For a long time, this metal was considered a type of antimony, lead or tin. The first information about metallic bismuth, its extraction and processing is found in the works of the largest metallurgist and mineralogist of the Middle Ages, George Agricola, dated 1529. The idea of bismuth as an independent chemical element developed only in the 18th century. The symbol Bi was first introduced into chemical nomenclature by the outstanding Swedish chemist Jens Jakob Berzelius.
There are several versions about the origin of the word “bismuth”. According to one of them, it is believed that it is based on the German roots “wis” and “mat” (distorted weisse masse and weisse materia) - white metal (more precisely, white mass, white matter). According to another, the word “bismuth” is nothing more than the Arabic “bi ismid”, that is, similar to antimony.
Bismuth - element 83 of the periodic table
Bismuth has been known since the 15th century, but it has long been mistaken for a form of tin, lead or antimony. In 1529, the German scientist in the field of mining and metallurgy G. Agricola gave the first information about metallic bismuth, its extraction and processing. The chemical identity of bismuth was first established in 1739 by I. Pott.
Presumably the Latin Bismuthum or bisemutum comes from the German weisse Masse, white mass.
The bismuth content in the earth's crust is very small and amounts to only 9·10-7% (71st place among all elements). In nature it is sometimes found in free form. Ismuth is a rare trace element and its own minerals are very rare. The most important of them are: bismuthin, or bismuth luster, Bi2S3 (81.3% Bi), cosalite Pb2Bi2S5 (42% Bi), bismite Bi2O3 (89.7% Bi) and some others.