The ounce is an ancient unit of weight that is not included in the international units of measurement. Nowadays, ounces are used less and less, mainly for two purposes: to measure the weight of metal and pharmaceutical ingredients. This unit of measurement has taken root and gained popularity not in all countries. And over time, another variety appeared - fluid ounce. Therefore, to convert ounces to kilograms, you need to use either a formula or online converters.
Before becoming a measure of weight, the ounce first appeared in Ancient Rome. This word was used to describe a coin that was made from an alloy of copper, tin and lead. One ounce was equal to 20 denarii. Coins with this weight were also minted in other European countries. But there was no concept of a fluid ounce.
Troy ounce
And Russia also had its own ounce. And most often it was used in pharmacies, where it was necessary to weigh powder or other medicine in solid form.
General information
Density is a property that determines how much of a substance by mass is per unit volume. In the SI system, density is measured in kg/m³, but other units are also used, such as g/cm³, kg/l and others. In everyday life, two equivalent quantities are most often used: g/cm³ and kg/ml.
Factors affecting the density of a substance
The density of the same substance depends on temperature and pressure. Typically, the higher the pressure, the more tightly the molecules are compacted, increasing density. In most cases, an increase in temperature, on the contrary, increases the distance between molecules and reduces density. In some cases, this relationship is reversed. The density of ice, for example, is less than the density of water, despite the fact that ice is colder than water. This can be explained by the molecular structure of ice. Many substances, when transitioning from a liquid to a solid state of aggregation, change their molecular structure so that the distance between the molecules decreases and the density, accordingly, increases. During the formation of ice, the molecules line up in a crystalline structure and the distance between them, on the contrary, increases. At the same time, the attraction between the molecules also changes, the density decreases, and the volume increases. In winter, you must not forget about this property of ice - if the water in the water pipes freezes, they can break.
Ice floats at the interface between water and less dense isopropyl alcohol, which is colored blue.
Ounces to Kilograms Calculator
You convert ounces to kilograms
Calculator - Weight and Mass - Ounces to Kilograms
How many kilograms in ounces - convert oz to kg
1 Ounce (oz) = 0.02 Kilograms (kg)
Ounces An ounce (abbreviation: "oz") is a unit of mass that has several definitions. Often an ounce is equal to 28 grams. Its size may vary in different systems. Today, the most widely used international ounces are avoirdupois, equal to 28.3495231 grams, and troy ounces, equal to 31.1034768 grams.
Kilograms The kilogram (symbol: “kg”), also known as kilo, is the basic measurement of mass in the International System of Units. Defined as a measure equal to the mass of the International Prototype Kilogram (IPK), it is related to the mass of almost one liter of water. It is the only basic unit of measurement that uses the prefix (“kilo”, symbol: “k”) as part of its name. The kilogram is very stable, which is really important, since 4 of the 7 basic units of the international system are directly related to the kilogram.
Conversion of units of Weight and Mass
Convert from
Convert to
oz
=
kg
| Basic units of weight | |
| Carat | car |
| Gram | gr |
| Kilogram | kg |
| Milligram | mg |
| Ounce | oz |
| Lb | lb |
| Ton | T |
| Other units of mass | |
| Assarion | assarion |
| Atomic mass unit (Dalton) | A. eat. |
| Attogram | ag |
| Bekah | |
| Centigram | cg |
| Dalton | |
| Dg | dg |
| Dekagram | dag |
| Denarius | denarius |
| Didrachm | didrachma |
| Drachma | drachma |
| Dina | dyn |
| Exagram | Eg |
| Femtogram | fg |
| Hera | gerah |
| Gigagram | Gg |
| Grain | gr |
| Hectogram | hg |
| Hundredweight | cwt |
| Hundredweight(UK) | cwt |
| Kip | |
| Mite | lepton |
| Megagram | Mg |
| Microgram | µg |
| Mine | mina |
| Mina(Biblical Hebrew) | mina |
| Nanogram | ng |
| Pennyweight | pwt |
| Petagram | Pg |
| Picagram | pg |
| Poundal | pdl |
| Quadrant | quadrans |
| Quarter | |
| Quarter(UK) | quarter |
| Quintal | quint. |
| Scruple | scr |
| Shekel | shekel |
| Slug | |
| Stone | st |
| Stone(UK) | st |
| Talent(Greek) | |
| Talent(Hebrew) | |
| Teragram | Tg |
| Tetradrachm | tetradrachma |
| Ton | |
| English ton | |
| Troy ounce | |
| Pharmacist's pound | |
| Basic units of weight | |
| Carat | car |
| Gram | gr |
| Kilogram | kg |
| Milligram | mg |
| Ounce | oz |
| Lb | lb |
| Ton | T |
| Other units of mass | |
| Assarion | assarion |
| Atomic mass unit (Dalton) | A. eat. |
| Attogram | ag |
| Bekah | |
| Centigram | cg |
| Dalton | |
| Dg | dg |
| Dekagram | dag |
| Denarius | denarius |
| Didrachm | didrachma |
| Drachma | drachma |
| Dina | dyn |
| Exagram | Eg |
| Femtogram | fg |
| Hera | gerah |
| Gigagram | Gg |
| Grain | gr |
| Hectogram | hg |
| Hundredweight | cwt |
| Hundredweight(UK) | cwt |
| Kip | |
| Mite | lepton |
| Megagram | Mg |
| Microgram | µg |
| Mine | mina |
| Mina(Biblical Hebrew) | mina |
| Nanogram | ng |
| Pennyweight | pwt |
| Petagram | Pg |
| Picagram | pg |
| Poundal | pdl |
| Quadrant | quadrans |
| Quarter | |
| Quarter(UK) | quarter |
| Quintal | quint. |
| Scruple | scr |
| Shekel | shekel |
| Slug | |
| Stone | st |
| Stone(UK) | st |
| Talent(Greek) | |
| Talent(Hebrew) | |
| Teragram | Tg |
| Tetradrachm | tetradrachma |
| Ton | |
| English ton | |
| Troy ounce | |
| Pharmacist's pound | |
Conversion result:
Other popular mass and weight calculators
- Carat to Gram
- Carat to Kilogram
- Carat to Milligram
- Carats to Ounces
- Carat to Pound
- Carats to Tons
- Gram to Carat
- Gram to Kilogram
- Gram to Milligram
- Gram to Ounce
- Gram to Pound
- Grams to Tons
- Kilogram to Carats
- Kilograms to Grams
- Kilogram to Milligram
- Kilograms to Ounces
- Kilogram to Pounds
- Kilogram to Tons
- Milligram to Carat
- Milligram to Gram
- Milligram to Kilogram
- Milligram to Ounce
- Milligrams to Pounds
- Milligrams to Tons
- Ounce to Carats
- Ounce to Gram
- Ounce to Kilogram
- Ounce to Milligram
- Ounce to Pound
- Ounce to Ton
- Pound to Carat
- Pound to Gram
- Pound to Kilogram
- Pound to Milligram
- Pound to Ounce
- Pound to Tons
- Ton to Grams
- Ton to Kilos
- Ton to Milligrams
- Ton to Ounces
- Ton to Pounds
Density of water
If the density of the material from which the object is made is greater than the density of water, then it is completely immersed in water. Materials with a density lower than that of water, on the contrary, float to the surface. A good example is ice, which is less dense than water, floating in a glass on the surface of water and other drinks that are mostly water. We often use this property of substances in everyday life. For example, when constructing ship hulls, materials with a density higher than the density of water are used. Since materials with a density higher than the density of water sink, air-filled cavities are always created in the ship's hull, since the density of air is much lower than the density of water. On the other hand, sometimes it is necessary for an object to sink in water - for this purpose, materials with a higher density than water are chosen. For example, in order to sink light bait to a sufficient depth while fishing, anglers tie a sinker made of high-density materials, such as lead, to the fishing line.
The density of fat is lower than the density of water, so it is easy to remove from the surface of soups, especially those cooled in the refrigerator to the temperature where the fat solidifies. It is convenient to remove them from both aspic and aspic, as in the photo. Photo published with permission of the author.
Oil, grease and petroleum remain on the surface of the water because their density is lower than that of water. Thanks to this property, oil spilled in the ocean is much easier to clean up. If it mixed with water or sank to the seabed, it would cause even more damage to the marine ecosystem. This property is also used in cooking, but not of oil, of course, but of fat. For example, it is very easy to remove excess fat from soup as it floats to the surface. If you cool the soup in the refrigerator, the fat hardens, and it is even easier to remove it from the surface with a spoon, slotted spoon, or even a fork. In the same way it is removed from jellied meat and aspic. This reduces the calorie content and cholesterol content of the product.
Information about the density of liquids is also used during the preparation of drinks. Multilayer cocktails are made from liquids of different densities. Typically, lower-density liquids are carefully poured onto higher-density liquids. You can also use a glass cocktail stick or bar spoon and slowly pour the liquid over it. If you take your time and do everything carefully, you will get a beautiful multi-layered drink. This method can also be used with jellies or jellied dishes, although if time permits, it is easier to chill each layer separately, pouring a new layer only after the bottom layer has set.
The cherry tomato floats on the boundary between the pink-colored salt water below and the lower-density fresh water above. The density of a tomato is greater than the density of pure water and less than the density of salt water, which is why it ended up in the middle.
In some cases, the lower density of fat, on the contrary, interferes. Products with a high fat content often do not mix well with water and form a separate layer, thereby deteriorating not only the appearance, but also the taste of the product. For example, in cold desserts and smoothies, high-fat dairy products are sometimes separated from low-fat dairy products such as water, ice and fruit.
Density of salt water
The cherry tomato floats on the boundary between the pink-colored salt water below and the lower-density fresh water above. The density of a tomato is greater than the density of pure water and less than the density of salt water, which is why it ended up in the middle.
The density of water depends on the content of impurities in it. In nature and in everyday life, pure H2O water without impurities is rarely found - most often it contains salts. A good example is sea water. Its density is higher than that of fresh water, so fresh water usually “floats” on the surface of salt water. Of course, it is difficult to see this phenomenon under normal conditions, but if fresh water is enclosed in a shell, for example in a rubber ball, then this is clearly visible, since this ball floats to the surface. Our body is also a kind of shell filled with fresh water. We are made up of 45% to 75% water - this percentage decreases with age and as we gain weight and body fat. Fat content of at least 5% of body weight. Healthy people have up to 10% body fat if they exercise a lot, up to 20% if they are of normal weight, and 25% or more if they are obese.
If we try not to swim, but simply float on the surface of the water, we will notice that it is easier to do this in salt water, since its density is higher than the density of fresh water and the fat contained in our body. The Dead Sea's salt concentration is 7 times the average salt concentration in the world's oceans, and it is famous around the world for allowing people to easily float on the surface of the water without drowning. Although, it is a mistake to think that it is impossible to die in this sea. In fact, people die in this sea every year. The high salt content makes the water dangerous if it gets into your mouth, nose, or eyes. If you swallow such water, you can get a chemical burn - in severe cases, such unlucky swimmers are hospitalized.
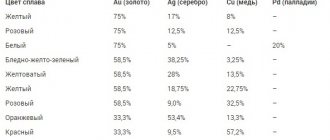
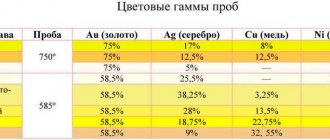
Varieties of ounces
Before converting one value to another, decide which ounce you are interested in. Ounce is abbreviated as oz. If you see this sign, be aware that you will need to convert ounces to grams to understand the weight of the item. Next to this value, another letter is assigned, depending on the type of ounce. There are several types:
- Troy ounce. Its value is 31.1034 grams. It is by far the most popular, since it is in it that the weight of gold is measured. This process takes place on the precious metals exchange in London. There, the measure of weight is the troy ounce, which, as a guarantor of stability, has accompanied gold for several centuries. A fluid ounce is not a troy ounce. The name originated in the town of Troyes, where fairs were held. Buyers and sellers could not agree and determine the value of coins or bullion bars. Therefore, money began to be equalized by weight. Therefore, this standard is nothing more than following traditions. They even issued several coins that weigh exactly the same as a troy ounce.
- An avoirdupois ounce, or trade ounce, corresponds to 28.35 grams. This is a type of trade ounce that was previously used even in the sale of groceries. Nowadays weight is measured in pounds. This is popular in the USA and England. One pound is the same weight as 16 ounces.
- A pharmaceutical ounce corresponds to approximately thirty grams of the substance.
Air density
The hot air inside this balloon is less dense than the surrounding air. This allows the ball to rise into the air and fly. Ruins of the ancient Mayan city of Teotihuacan, Mexico.
Just as in the case of water, bodies with a density lower than the density of air have positive buoyancy, that is, they take off. A good example of such a substance is helium. Its density is 0.000178 g/cm³, while the density of air is approximately 0.001293 g/cm³. You can see helium soar in the air if you fill a balloon with it.
The density of air decreases as its temperature increases. This property of hot air is used in balloons. The balloon in the photograph at the ancient Mayan city of Teotihuocan in Mexico is filled with hot air that is less dense than the surrounding cold morning air. That is why the ball flies at a fairly high altitude. While the ball flies over the pyramids, the air in it cools down and is heated again using a gas burner.
A little history
The term "ounce" originated in ancient Rome. This universal value could give an idea of length, surface, capacity or monetary equivalent. In medieval Europe it was firmly established as a unit of measurement of weight.
In the modern world, it has come into use in countries where it is customary to measure cargo in pounds, for example, England.
Its prototype is also used, which is called Troy, it is widely used in commerce for precious metals. There are also the following subtypes - liquid, apothecary and avoirdupois.
Density calculation
Often the density of substances is indicated for standard conditions, that is, for a temperature of 0 °C and a pressure of 100 kPa. In educational and reference books you can usually find such densities for substances that are often found in nature. Some examples are shown in the table below. In some cases, the table is not enough and the density must be calculated manually. In this case, the mass is divided by the volume of the body. The mass can be easily found using a scale. To find out the volume of a body of a standard geometric shape, you can use formulas to calculate volume. The volume of liquids and solids can be found by filling a measuring cup with the substance. For more complex calculations, the liquid displacement method is used.
Liquid displacement method
To calculate the volume in this way, first pour a certain amount of water into a measuring vessel and place the body whose volume needs to be calculated until it is completely immersed. The volume of a body is equal to the difference in the volume of water without the body and with it. It is believed that this rule was derived by Archimedes. Volume can be measured in this way only if the body does not absorb water and does not deteriorate from water. For example, we will not measure the volume of a camera or fabric product using the liquid displacement method.
It is unknown to what extent this legend reflects actual events, but it is believed that King Hiero II gave Archimedes the task of determining whether his crown was made of pure gold. The king suspected that his jeweler had stolen some of the gold allocated for the crown and instead made the crown from a cheaper alloy. Archimedes could easily determine this volume by melting the crown, but the king ordered him to find a way to do this without damaging the crown. It is believed that Archimedes found the solution to this problem while taking a bath. Having immersed himself in water, he noticed that his body had displaced a certain amount of water, and realized that the volume of displaced water was equal to the volume of the body in the water.
Hollow bodies
Some natural and man-made materials are composed of particles that are hollow, or particles so small that they behave like liquids. In the second case, an empty space remains between the particles, filled with air, liquid, or other substance. Sometimes this place remains empty, that is, it is filled with a vacuum. Examples of such substances are sand, salt, grain, snow and gravel. The volume of such materials can be determined by measuring the total volume and subtracting from it the volume of voids determined by geometric calculations. This method is convenient if the shape of the particles is more or less uniform.
For some materials, the amount of empty space depends on how tightly the particles are packed. This complicates calculations because it is not always easy to determine how much empty space there is between particles.
Table of densities of substances commonly found in nature
| Substance | Density, g/cm³ |
| Liquids | |
| Water at 20°C | 0,998 |
| Water at 4°C | 1,000 |
| Petrol | 0,700 |
| Milk | 1,03 |
| Mercury | 13,6 |
| Solids | |
| Ice at 0°C | 0,917 |
| Magnesium | 1,738 |
| Aluminum | 2,7 |
| Iron | 7,874 |
| Copper | 8,96 |
| Lead | 11,34 |
| Uranus | 19,10 |
| Gold | 19,30 |
| Platinum | 21,45 |
| Osmium | 22,59 |
| Gases at normal temperature and pressure | |
| Hydrogen | 0,00009 |
| Helium | 0,00018 |
| Carbon monoxide | 0,00125 |
| Nitrogen | 0,001251 |
| Air | 0,001293 |
| Carbon dioxide | 0,001977 |
Density and mass
Airplanes often use composite materials instead of pure metals, since, unlike metals, such materials have high elasticity and low weight. The propellers of this Bombardier Q400 aircraft are made entirely of composite materials.
Some industries, such as aviation, require materials that are as light as possible. Since low-density materials also have low mass, in such situations they try to use materials with the lowest density. For example, the density of aluminum is only 2.7 g/cm³, while the density of steel is from 7.75 to 8.05 g/cm³. It is due to the low density that 80% of aircraft bodies use aluminum and its alloys. Of course, one should not forget about strength - today few people make airplanes from wood, leather, and other lightweight but low-strength materials.
Airplanes often use composite materials instead of pure metals, since, unlike metals, such materials have high elasticity and low weight. The propellers of this Bombardier Q400 aircraft are made entirely of composite materials.
An artistic depiction of a black hole by NASA.