Noble metals have been known to people since ancient times. They were used to make jewelry and weapons for high-ranking officials, rulers, and military leaders. Gold is considered a beautiful material with unusual qualities. To expand its applications, jewelers began to create mixtures with the addition of other materials. An alloy of gold and silver is used in various industries.
Alloy of gold and silver
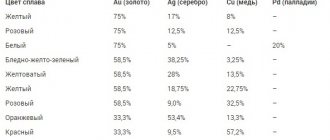
Color of gold and silver alloy according to GOST
One of the main features of electrum is its color spectrum of greenish-yellowish tones. This quality is reflected in regulatory documents such as GOST. Samples used to mark the alloy: 585 (14 carats), 750 (18 carats).
Gold leaf, which consists of thin plates for gilding various objects, architectural structures, such as church domes, has a fineness of 960 (23 carats).
The color of green precious metal according to Gosstandart is described by the following compounds:
585 sample:
- ZlSR 585 -415 – green color, melting point 1025-1030 degrees;
- ZlSrM 585-300 - yellow-green color, melting point 835-880 degrees.
Properties and structure
There has long been a concept that a compound in which silver predominates (more than 70%) is it. The same was said about other components. Electrum occupied a middle position between these metals. Current realities show that it is possible to produce products that contain less than 35% gold. Depending on the content of components in the composition, the properties of elethrum also change:
- belongs to the class of native elements;
- increased resistance to oxidation;
- high melting point;
- density - 6.5 g/cm3;
- Mohs hardness - 3 points.
The properties of the composition are not precisely described. They vary depending on the percentage of mixture components.
Range of alloys of gold with other metals in the jewelry industry
Along with electrum, which combines gold and silver into an alloy, there are a number of compounds that are used to make coins and jewelry. The most common ones are characterized by the following samples:
- 999 – 24-carat, less than 0.1% impurities. It is used primarily for the production of ingots. For other purposes it is of little use due to its increased softness.
- 916 – 22 carats. Used mainly in the east.
- 750 – 18 carats. High quality alloy with 75% gold.
- 585 – 14 carats. The most popular standard, widely used by jewelers.
- 375 – 9 carats. A low-grade precious metal, usually alloyed with copper, so it has a reddish tint. Products made from it are found quite often.
In addition to these samples, there are a number of rarer ones, products with which are used in industrial production. They are not used to create jewelry.
In Tsarist Russia, other samples were used and can be found on antique items made of precious metals. They were marked: 56, 72, 82, 92, 94, 96, measured by spools. This is an ancient measure of weight - 4.27 grams; it corresponded to a weight of the same mass.
Alloys with gold make this metal more wear-resistant and practical
Subtleties of alloy manufacturing
When making an alloy, you need to take into account some features:
- Melting of gold and other noble materials must occur before additional components. This is due to their rapid oxidation in air.
- If you add copper before noble materials, the mixture will harden unevenly, which will negatively affect its further processing and operation.
- The percentage of additional components affects the color of the electrum and its characteristics.
- To prevent the mixture from darkening, you need to take 3 parts gold and 1 additional component.
It is important to control the temperature during melting. This will prevent the metals from burning through.
After production, the craftsman will want to know the sample of the resulting product. To do this, you need to find out its total mass and the weight of individual components. If there is 60 grams of gold, and the product weighs 100, then it can be classified as 585.
Alloy making
The popularity of the alloy of gold and silver in ancient times, in various industries today
Known since ancient times, electrum has found wide application. Due to its unique qualities and unusual color, it has been used in various ways.
Ritual weapons
Weapons for rulers and military leaders of the Ancient world could contain inserts made of an alloy of gold and silver.
In Ancient Egypt, they covered obelisks, the tops of pyramids, the so-called pyramidia.
Weapons of Ancient Egypt Warriors
Valuable jewelry
Products made of unusual whitish and greenish precious metal have been popular since ancient times.
Nobel Prize medals have been molded from 100% electrum for decades.
First minted coins
Archaeologists found the earliest coins in the territory of the Ancient Kingdom of Lydia, Eastern Greece. They were called Lydian coinage after their geographical name. Their use was due to their high hardness and wear resistance, better than that of the base noble metal.
Historical data
Precious alloys of these metals have been known since antiquity. The ancient Greeks gave the compound the name “electrum”, but they began to mine it in ancient Lydia. The silver content could reach 50%. Because of this, the color of the product changed to milky yellow. Therefore, many people call this compound “white gold.”
Technologies make it possible to create a mixture artificially. The composition may consist of 70% silver. The color is similar to the predominant component.
Application
Alloys of precious metals are used in the jewelry industry , where they are used to make various jewelry, such as rings, chains, pendants and earrings. In dentistry , they are used in the production of bridges and crowns.
In the chemical industry, certain alloys are needed to coat pipes that are used to transport aggressive chemicals. In the electronics industry, there is a need for gold-silver alloys for the manufacture of current-collecting electrodes. In microelectronics, noble metal alloys are used in the form of electroplating of connectors and contact surfaces. They are also used for the manufacture of valuable award paraphernalia in sports (cups, medals). In the nuclear field, it is also impossible to do without precious metals and their mixtures.
Based on these substances, even pharmacological drugs are produced to combat diseases such as tuberculosis or rheumatoid arthritis.
Products made from an alloy of yellow metal will always be in demand , because, in addition to all of the above, they are also a good investment for the future.
Learn more about another popular alloy of precious metals – red gold – in the video below.
Who can wear silver?
First of all, these are girls and women with the “winter” and “summer” color types. People with this appearance have light, porcelain- or ivory-colored skin and light, brown, or dark hair. Silver jewelry will also look great on women with gray hair.
Interesting materials:
What is the chemical bond in Hydrogen Bromide? What acid is contained in nettle? Which key allows you to enable an additional keyboard? Which team became the first women's basketball world champions? What is the commission for Delivery? Which company produces the Lord of the Rings series? What is the composition in the comedy The Inspector General? What kind of skin does a polar bear have? Which noodles are healthier? What is the maximum computer RAM?
Ancient scriptures
The chronology has such a period as millennia BC. Many people know about this time from historical books that describe the reign of the Egyptian pharaohs. Back then, noble people on the street were distinguished from the poor by the fact that the rich wore gold jewelry.
The first nuggets of this mineral were found in East Africa on the banks of the Nile River, where Egypt is also located. Electrum was originally used to make Lydian money, as pure gold is much heavier and more fragile. And this alloy was distinguished by its lightness and wear resistance due to the presence of silver in it. It was also soft enough and amenable to forging, so they also made dishes from it. It did not oxidize, unlike iron, and was often found during excavations of the burial places of the pharaohs. The famous Cleopatra was very fond of jewelry made from this alloy.
In Ancient Egypt, alchemists who studied astrology knew 8 minerals. Among them were gold, silver, iron, copper, tin, lead, emerald and electrum. Therefore, each planet had its own metal assigned. In ancient times, the week began with Sunday, which is ruled by the Sun, and, of course, its element is gold. Monday is ruled by the Moon and holds silver. The iron was given to Mars. Mercury is associated with tin and Venus with copper. Heavy lead is attributed to Saturn.
Jupiter is considered the planet of wealth, so it rightfully received electrum, which combines three minerals at once. This can be learned from the manuscript, which is currently kept in the library of St. Mark, located in Venice.
The main rules for choosing jewelry made of precious metals
For every woman, having accessories made of noble material allows you to emphasize the sophistication of taste, elegance and is a way to clearly express your unique style.
When selecting jewelry, you should adhere to the following basic rules:
- do not use accessories that will look cheaper than clothes;
- do not combine opposing styles (jewelry looks bad against the background of sportswear);
- closely follow fashion, but choose jewelry individually;
- do not violate the rule of three (permissible combination of no more than three design elements);
- select the shape of the products taking into account the physique and figure;
- fragile bracelets are perfect for slender girls;
- To emphasize the earrings, you should use the color play of stones.
People wear jewelry for various reasons. For some, jewelry emphasizes the level of wealth that makes it possible to buy jewelry from famous brands, while for others, jewelry is a talisman that brings good luck. But is it possible to wear jewelry made from different materials at the same time?
Mineral electrum AuAg
Mineral species of variable composition from the extreme member of silver - Ag to the extreme member of gold Au. Both varieties usually contain impurities of copper and bismuth. In addition, impurities of antimony and mercury are found in silver, and palladium in gold. The structural cell contains 4Ag, 4Au. Space group O⁵ h
- Fm3m.
Aggregates and habit.
Silver is observed in the form of thin plates, leaflets and “knitted” dendrites. Various wire forms are very common. Silver is mainly found in the form of irregular grains and large continuous accumulations - nuggets. In the Schneeberg deposit in Saxony, a silver nugget weighing 40 tons was found, and in Freiberg - 5 tons. In Chile, plates of native silver weighing about 1.5 tons were found. Dendrites and “knitted” forms are characterized by the development of individuals along quadruple axes, resulting in between the branches right angles and 60° angles are formed. Dendrites with branches developed at right angles are simple individuals, and with branches developed at an angle of 60°, they are twin intergrowths according to (111). Silver is very rare in the form of crystals. Silver crystals usually have a cubic, octahedral and, much less frequently, dodecahedral habit; the main shapes on them are the cube {100}; octahedron {111} and rhombic dodecahedron {PO}. Twins at (111) are marked. Repeating themselves, they form radiant aggregates and dendrites. Parallel intergrowths of octahedral individuals are very common. Gold is mainly observed in the form of small irregular grains, flakes and plates embedded in the rock, which are often difficult to distinguish even under a microscope. In the placers of river valleys, rounded gold nuggets are found, weighing from several grams to several tens of kilograms. The largest nugget “Holterman Plate” (weight with rock 260 kg, net weight - 93.3 kg) was found at the Hill End mine in Australia in 1872. The largest pure gold nuggets “Pleasant Stranger” (weight 59.67 kg, 1857), “Welcome Guest” (weight 68.08 kg, 1869) - also found in Australia, in the state of Victoria.
Gold crystals are rare. They have a predominantly octahedral and rhombic dodecahedral habit and rarely a cube shape. Sometimes the rhombic dodecahedron and crystals turn out to be elongated along the quadruple axis and have an elongated appearance. The edges of the gold crystals are covered with various sculptures and shading. Often gold crystals are twinned according to the spinel law according to (111) or have the appearance of tree-like formations and mesh plates, which are intergrowths composed of small individuals.
Diagnostic signs. Silver is determined by the nature of the aggregates, color, hooked splinter fracture, malleability and density. Main lines on radiographs: 2.37; 2.05; 1.232. It dissolves in HNO3, as well as in HCl, releasing a characteristic white cheesy precipitate AgCl. H2S turns it black. P. p. t. silver melts (melting point is close to 960 C.)
The most characteristic properties of gold are its golden yellow color, good malleability, high density, non-oxidization in air and low hardness. Main lines on radiographs: 2.35; 2.03; 1.226. It does not dissolve in acids, with the exception of aqua regia. P. p. t. melts (melting point 1062°) .
Differences from similar minerals. Silver differs from platinum in its lower density and lower hardness (density of platinum is 21.45, hardness is about 4), as well as in the nature of the aggregates. In addition, unlike platinum, silver is never found as inclusions in igneous rocks. Silver-like argentite (Ag2S) is a darker lead-gray or black color. Gold differs from pyrite (Fe[S2]), alcopyrite (CuFeS2) and millerite (NiS) in its strong luster, characteristic color shade, hardness, malleability, and insolubility in acids. Artificial acquisition. Silver is reduced from solutions of its salts by organic and inorganic reducing agents, as well as by electrolysis. Gold crystals were obtained by the action of high temperature or acids on gold amalgam, as well as by reduction from a solution of gold salts and by electrolysis.
Decoding the markings of alloys that contain gold
Materials are marked for ease of handling. For precious metals, in addition to the letter, an additional numerical designation is generally accepted - fractions of thousandths, and for base alloys - hundredths (%). Common options found in GOST:
- gold – Evil;
- silver - Wed;
- platinum – Pl;
- copper – M;
- zinc – C;
- nickel – N;
- cadmium – Kd;
- palladium – Pd.
Medical gold alloy
COLORED GOLD
Black, blue or purple gold, which has a vibrant and rich color, is created on the basis of intermetallic compounds, which are not alloys. All of these compounds, although metallic in appearance, have a fixed composition, are very brittle, resemble ceramics, and present significant processing difficulties. The buyer will not be able to change the size of such a ring, change its shape, and it will not be accepted either at the pawn shop or at the buying site. When purchasing such products, I recommend taking this into account!
To give gold alloys a black color, the following technological method is usually used: the surface of the jewelry is coated with a layer of black rhodium or ruthenium using the galvanic method; the color of the coatings varies from gray to black. But, like any coating, it is short-lived and will wear off during use.
Usage
Electrum has been used since the third millennium BC. e. (in the Ancient Kingdom of Egypt for covering obelisks and pyramid tops). The first mention is found in the description of the expedition sent by Pharaoh Sakhur from the V dynasty. There is also a mention in Pliny the Elder in his Natural History.
The first coins in history were made from electrum (in Lydia in the 7th century BC). Electrum was convenient for making coins because it is harder than gold and therefore more wear-resistant. In addition, the technology for refining gold was undeveloped at that time.
The first method of analyzing electrum for gold content apparently belongs to Archimedes (Archimedes’ famous discovery of a method for measuring density, made “in a bath”); before that, the exchange rate for electrum coins was determined by agreement [2].
World systems for measuring the purity of gold precious metals
At different times, a wide variety of methods were used to determine the Au content in a metal alloy. At the same time, the number of ligatures that were added at the manufacturing stage varies: from 10-12% to 80%. But the best option in terms of appearance and properties is the one that contains 30-40% additives. There are only 4 known ways to measure the purity of a precious metal:
- widely used metric system: the fraction of the precious metal is determined in thousandths (range from 0 to 1000);
- carat system, applicable in the USA and Europe: the value taken as a basis is 24 carats, this is a precious metal without impurities; accordingly, the gold content in different alloys is determined as the number of parts of 1/24 of the pure substance (corresponding to 1 carat);
- spool system of Tsarist Russia: the basis was taken as a measure - 96 samples of pure precious metal, corresponding to the number of spools in 1 Russian pound;
- lot system of Old Europe: metal of 16 lots was considered pure; the lower this number, the lower the value of the products.
See also[edit]
- Corinthian bronze was a highly prized alloy in antiquity that may have contained electrum.
- Hepatizon
- List of alloys
- Orichalcum is another special metal or alloy mentioned in texts of classical antiquity, later used to refer to brass.
- Panchaloha
- Shakudo is a Japanese billon made of gold and copper with a dark blue-violet patina.
- Shibuichi is another Japanese alloy known for its patina.
- Thokcha is an alloy of meteoric iron or "thunder iron" commonly used in Tibet.
- Tumbaga is a similar material originating from pre-Columbian America.
Jeweler's comment
Molokanov N.M.
Jeweler, 26 years of experience in jewelry production.
The content of the alloy makes the metal different from others. Today, the composition is often changed, according to GOST, as a result of a decrease/increase in the proportions of gold, copper, silver or other metals, which can affect strength, density, fusibility and, of course, color. Thanks to this feature, the shade of precious metals serves as one of the signs of authenticity.
Natural reserves of electrum
There is reliable information that electrum was brought to Egypt from Punt. In the modern world, this is East Africa. Another supply was established from the territory of the Plateau and to the east of Rhodesia.
Pliny gave a detailed assessment of the precious materials of that time. He said that if the composition contains more than 70% of a white metal, it will be silver, if less than 15%, it will be gold.
Electrum was also mentioned in the Odyssey. There he was mentioned in conversations about gold jewelry plated with silver. Today, these products are not relevant, but then they were in great demand, since white metal was sometimes valued more than gold.
The electron-rich lands (another name for the composition) of that time are no longer so. The metal, which was practically on the surface, had been collected long ago. To a greater extent this happened during the period of “fever”. Therefore, humanity began to think about obtaining it artificially, because it is no longer possible or extremely difficult to find it in nature.
It can only be found in Western Anatolia. This is one of the areas located in Turkey. But its extraction has already lost popularity due to the well-established technology for producing electrum on its own; moreover, the natural material may have an undesirable admixture of copper. While with the manual method you can avoid such situations and do not devote time to freeing the alloy from unnecessary contaminating materials.
Varieties
Native gold has several varieties.
- Electrum (or silver gold).
- Cuproaurite (or auricupride), otherwise called cuprous (pure) gold. Contains, in addition to the yellow metal, a significant percentage of copper. At a copper content of 9-20% the color becomes pinkish, at 25-30% it becomes red. Because of this color, the name of such nuggets came about - red gold.
- Bismuthaurite (or bismuth gold). Contains up to 4% bismuth.
- Will give birth (born gold). Contains up to 43% rhodium.
- Porpecite (palladite gold). Named after the Porpetse region in Brazil, it contains between 5% and 12% palladium. The nugget is white.
- Iridescent gold. Contains up to 30% iridium in pure form or with impurities.
- Platinum gold. This alloy contains up to 11% platinum or a mixture.
- Magnetic gold. Contains iron impurities.
In our country, electrum is mined in the Far East (Khakanja deposit), Aldan, the Urals and Altai, Transbaikalia, the Republic of Sakha (Yakutia) and Primorye.
Effect of impurities
Among the alloys, which is the name given to the impurities that are used in gold alloys, there are quite traditional, time-tested metals. It is worth mentioning right away that the alloy that is used in Russia for the production of jewelry can only inspire confidence when it is made in accordance with GOST. A special commission studied various tests for their strength, wear resistance, hypoallergenicity and established standards that all official manufacturing companies must comply with. Alloys of 375, 500, 585, 750, 958, 999 samples were approved. The 583 sample was excluded after the collapse of the USSR and replaced by the 585 sample. Any intermediate indicators can only be found in products imported from abroad.
Among the traditional additives of jewelers are silver, cadmium, platinum, copper, palladium, nickel, and zinc. Let's consider the properties that certain components impart to the metal.
Silver is the base version of the ligature for many types of gold. Silver lowers the melting point and makes the alloy more malleable. It also adds strength to the finished product. Its content in the product can range from a very small percentage to 60-65%. First of all, the percentage of content will affect the appearance of the product, namely the color of the metal. The more silver there is, the less yellowness there is in the metal. An alloy with 60 percent or more silver becomes white. While in Russia they prefer the addition of copper, in Europe they mainly use silver, so gold differs from the jewelry houses of European capitals in its characteristic light lemon tint.
Copper is an attribute of Soviet gold. And now its use as a ligature is widespread. It gives hardness and a reddish tint to gold alloys. Often, in addition to copper, at least 5% silver is added, since its high content reduces the anti-corrosion properties of the alloy. The alloy, beloved by residents of the CIS, however, is not respected in many countries, for example, in the UAE such gold is simply considered to be of poor quality.
Platinum is an expensive metal, so its addition affects the cost of the product. It supports gold in the fight against corrosion and gives the metal its whiteness. Its percentage is usually small, because to increase the elasticity of the alloy and its white color, less than 10% of the total mass of platinum is needed.
Palladium is a fairly rare alloy for gold alloys. Its advantage is to increase the melting point of gold and impart a white color. The palladium content can be about 10%, and this is already enough to change the color. It is rarely used as it is a refractory metal and difficult to work with.
Zinc is an alloy that changes the color of gold, giving it a green tint. In addition, it lowers the melting point and makes the alloy more fluid. Too high a percentage of zinc content can lead to brittleness of the alloy, but this option is acceptable for those elements of jewelry that are used as a decorative insert.
Appearance of zinc.
Cadmium is an element that is usually added in very small quantities. It can affect the color of the alloy, making it pale yellow or even gray, or it can only add special properties - lower the melting point.
Nickel is an alloy that makes the metal harder, allowing you to work with it more creatively, without worrying about the model’s resistance to pressure and shock. It practically does not change the color, only making the shade of yellow a little paler.
Alloys of gold with tin, lead, bismuth, and antimony are also acceptable, but they are not suitable for jewelry as they make the metal more fragile. Ruthenium, iridium and osmium can be added to strengthen the alloy, but they do not affect its color in any way.
Links[edit]
- ^ ab Chisholm, Hugh, ed. (1911). "Electrum, Electron". Encyclopædia Britannica
.
9
(11th ed.). Cambridge University Press. paragraph 252. - Emsley, John (2003) Nature Building Blocks: An A-Z Guide to the Elements. Oxford University Press. ISBN 0198503407. item 168
- Kurke, Leslie (1999). Coins, Bodies, Games and Gold: The Politics of Meaning in Archaic Greece. Princeton University Press. pp. 6–7. ISBN 0691007365.
- ^ abc Konuk, Koray (2012). Metcalf, William E. (ed.). Asia Minor before the Ionian Revolt. The Oxford Guide to Greek and Roman Coinage
. Oxford University Press. pp. 49–50. ISBN 9780199372188. - Cahill, Nick; Kroll, John H. New Archaic Coins Found at Sardis, AJA 109 (2005). pp. 609–614.
What causes metal to tarnish?
There may be several reasons for changing the appearance of a new ring:
- The new product was coated with a polishing compound. In this case, the skin on the finger will also darken. Contamination is easily removed.
- Unfortunately, sometimes a change in the color of a ring may indicate a low content of precious metal. We already know how easily inexpensive alloys can be passed off as jewelry.
- Body jewelry can respond to various changes in the body. Such stains from good jewelry can be easily cleaned.
- Gold deteriorates and becomes stained when exposed to mercury and iodine. We come into contact with the first substance as a last resort, but no one is safe from adding iodine to the water of a public swimming pool. When going to the pool, it is better to take off your jewelry, even if it is 375 standard.