Palladium
- a rare mineral, a noble metal of the platinum group, silver in color, not tarnished in air. Discovered by the English chemist and mineralogist W.H. Wollaston, who discovered palladium in native platinum in 1803. Malleable and malleable. More fusible compared to platinum, it is easily rolled and drawn into wire. Melting point 1552°C. Paramagnetic Soluble in HNO3, hot concentrated H2SO4 and aqua regia. Palladium has an extremely high affinity for hydrogen; in powder form it can absorb a volume of hydrogen 900 times greater than the metal’s own volume. Compared to other platinum metals, it is less resistant to oxidizing agents.
- Structure
- Properties
- Reserves and production
- Origin
- Application
- Classification
- Physical properties
- Optical properties
- Crystallographic properties
See also:
Silver
– structure and physical properties
What is palladium
The chemical element is a plastic mineral having a silvery-white color. It is classified as a type of precious metal of the platinum group.
Brief history of appearance
Pd was first discovered in the 19th century. The chemical element was discovered by chemist William Wollaston (Great Britain). During the experiments, the scientist extracted it from platinum ore.
The metal received its name in honor of the asteroid Pallas discovered a year before. He, in turn, was named so thanks to the goddess from Ancient Greece Pallas Athena and her wooden image, the Palladium, which fell from the sky according to legend.
Hoax in the world of platinum metals
The story of our hero's appearance in the human world is almost a detective story.
In 1803, Forster, a respectable mineral merchant, was brought a package with a letter. The letter contained a request to sell three ingots of metal, which (as they assured in the letter) was in no way inferior to platinum. The merchant knew a lot about advertising, took advantage of them, and passions flared up in London. The excitement was skillfully fueled by the Secretary of the Royal Society, W. G. Wollaston.
A certain Chenevix bought the ingot and spent a long time analyzing it; they showed that it was an alloy of platinum and mercury. Other chemists and mineralogists did not find these metals in the ingots.
When the hype died down, an advertisement was published in Nicholson's Journal. The author promised a substantial sum to anyone who synthesizes artificial palladium within a year. Interest in the bars arose again, but the prize remained unclaimed.
And in 1804, Wollaston reported that the hoax with the new metal was his doing. At this time, he was already convinced that palladium was a hitherto unknown element.
William Wollaston (1766–1828)
What does palladium look like in nature?
Nuggets are not found in nature in their pure form. Metal particles are extracted along with other minerals. According to approximate data, there are approximately 30 elements that come into contact with palladium.
Externally, grains of the precious metal are very similar to platinum. In some deposits, these two elements are mined together (called palladium platinum) and then separated by chemical processing. Also, veins can intersect with gold, then a combination of two metals is observed (for example, palladium gold or porpecite from Brazil).
The process of formation in nature
The main source of appearance is space fragments of meteorites. A large content of precious metal crystals was found in iron and stone types of alien fragments.
Structure, chemical and physical properties
By its nature, the mineral compares favorably with other precious metals with its low density and chemical inertness. Thanks to the latter property, it does not interact with other elements and does not oxidize.
Reactions:
- Exceptions are silicon, boron, sulfur, chromium, with which palladium forms chemical compounds.
- Also, metal crystals dissolve in “regia vodka” (this is a mixture of two acids - sulfuric and nitric).
Expert opinion
Vsevolod Kozlovsky
6 years in jewelry making. Knows everything about samples and can identify a fake in 12 seconds
In appearance, the nuggets are similar to platinum and silver. The metal is very ductile, which is why it is actively used in jewelry. To improve strength and wear resistance, it is used in compounds with other metals.
The melting point is 1554 degrees Celsius.
Jewelry and investment
Jewelers use palladium as an independent precious metal, as well as a component of other precious alloys. Pd is a component of the platinum master alloy, and is also almost always present as an element in the 585 and 750 gold alloys. All known and quite popular white gold today owes its hue to the addition of this particular chemical element. The precious metal itself is not used in the production of jewelry in its pure form; in high grades, ruthenium is present in the alloy alloy.
For global palladium production, the demand of the jewelry industry is not critical. Even despite the policy of popularizing palladium jewelry, consumer interest in them is low. Jewelry factories offer their customers both traditional jewelry for women - earrings, rings, brooches, pendants, and men's signets, crosses and cufflinks. The precious metal has been actively used in recent years in the collections of famous fashion designers, presenting not only jewelry, but also stylish and unusual accessories: watches, lighters, pens, wallets.
Where else is precious metal used? Palladium, due to its noble origin, is one of the investment instruments. You can invest in precious metals in different ways; among the available options for ordinary bank clients are purchasing bullion and opening a metal account. Compulsory medical insurance is carried out in grams of “virtual metal”, so when you open it you will not receive a single gram of Pd in your hands. But if you decide to purchase an ingot, you will be able to feel the weight of the metal in your hands.
Palladium in the precious metals market is considered one of the fundamental assets that can bring profit only in the long term. Most investments in the metal are futures contracts on the exchange; there is no point in buying palladium bars in order to increase your money. For novice investors and ordinary bank clients, it will be easiest to open an impersonal account. Another unique investment instrument directly related to Pd is commemorative coins. Coins from this metal are minted rarely and in limited quantities, so it is not practical to consider them as a source of profit.
How are palladium veins found?
Mineral inclusions are sought primarily in the locations of silver, copper and nickel ores. Occasionally there are small deposits with nuggets of pure metal.
Palladium satellites
In the bowels of the earth, palladium is found exclusively in the form of compounds with other minerals. Some of them have been little studied to this day and have no name. The most famous satellites of the precious metal are:
- braggite;
- palladite;
- potarit;
- stannopalladite.
It is also often extracted from gold and platinum veins.
Where does palladium occur in nature?
Under natural conditions of the earth's interior, the mineral is found in the form of compounds of different metals. Similar veins are found in Europe, the Russian Federation, and America.
ORIGIN
Palladium occurs as an impurity in many sulfides and silicates of ultramafic and mafic rocks. Some coals are enriched with palladium up to 10%; increased concentrations are observed in manganese ores, phosphorites, and plant ash. Palladium content is elevated in ultramafic rocks and rocks containing Cu, Ni and Te sulfides. Usually found in nature as an impurity in native platinum, with which it forms a disordered solid solution; sometimes found in its placers in the form of rounded grains. As a rule, it contains impurities of platinum, iridium, gold, and silver. Palladium platinum contains 19-40% palladium, palladium stannoplatinum -17-21%, polyxene - up to 6%, ferroplatinum - up to 13%, iridium platinum - up to 4%. It can also be found as an admixture to native gold (in Brazil, for example, a rare variety of native gold (porpecite) was found, containing 8-11% palladium). It is formed in the zone of oxidation of primary sources of platinum and directly in placers as a result of supergene transformation of platinum minerals. In iron meteorites, there is up to 7.7 grams of substance per ton. palladium, in stone - up to 3.5 g. Since alluvial deposits of native palladium are very rare, the main raw materials for its associated production are sulfide ores of nickel and copper (Norilsk region, etc.)
Extraction methods
Work with palladium deposits is carried out in two forms:
- closed (mine);
- open (career).
In the first case, a system of underground tunnels - mines - is created for the extraction of precious metals. Small holes are created in the found ore layer, into which explosives are then placed. The soil loosened by the explosion is processed mechanically or manually to extract palladium particles. Once the initial refining is complete, the ore is transported to the surface and then transported to further processing.
In the second case, heavy earth-moving equipment and vehicles are used to transport the extracted ore. With its help, a soil quarry is developed, from which palladium is then extracted. It is then transported for processing to the appropriate enterprises.
Areas of application
The element widely uses:
- Chemical industry. Pd is a popular catalyst in oil refining and fat refining. Palladium chloride is also involved in the search for trace amounts of carbon monoxide in the air or gas mixtures. In electrochemistry, the same compound is an activating substance in the galvanic metallization of dielectrics. Palladium membranes are needed for hydrogen purification.
- Electrical engineering. The metal is important as a coating that is resistant to sulfides: the manufacture of high-precision voltagometers. Its physical characteristics have led to its use in the production of ceramic capacitors.
- Jewelry making. Palladium is added to products to create white gold. Even a small metal content in the alloy changes the shade of the item from yellow to silver-white. Occasionally, the mineral is used in the manufacture of commemorative coins.
- Medicine. Palladium is added to drugs intended to combat tumors and cancer therapy. Another area where metal is used is dentistry. Here, dentures are made on its basis. Alloys with the addition of palladium are used to create individual parts of pacemakers and medical instruments.
Content
- 1. History
- 2 Origin of the name
- 3 Finding in nature 3.1 Obtaining
- 3.2 Production indicators
- 4.1 Isotopes
- 6.1 Catalysts
The richest deposits
Although large amounts of palladium are found in pieces of meteorites that fall to earth, the bulk of production comes from ore deposits. They provide about 98% of the world's metal reserves.
In the world
The Bushveld Complex (South Africa) is the world's largest palladium deposit. Here, prospectors find up to 40% of the world's precious metal reserves.
In much smaller quantities it is also extracted into:
- Lac des Iles (Canada);
- Stillwater (USA);
- Great Dike (Zimbabwe).
In Russia
The copper-nickel deposits that are part of OJSC MMC Norilsk Nickel are the largest metal suppliers in Russia:
- Oktyabrskoe;
- Talnakhskoe;
- Norilsk-1.
Their total profit is more than 40% of the global one.
Being in natural conditions
In terms of content in the earth's crust, this precious metal is one of the rarest elements. In nature it can be found in the form:
- nugget (allopalladium);
- minerals of the intermetallic type (stannopalladinite, palladium platinum, etc.);
- various compounds (braggite, palladinite, etc.).
There are about three dozen varieties of palladium minerals known to science. This element often acts as an accompaniment to other platinum metals. The content of the substance in the platinum mixture ranges from 25-60% (depending on the deposit).
Native palladium is extremely rare. In appearance it is almost indistinguishable from platinum. In addition to palladium, nuggets also contain other elements:
- iridium;
- gold;
- platinum;
- silver.
According to Goldschmidt's geochemical classification of elements, palladium (along with all other platinoids) is among the siderophiles. In other words, it has a characteristic affinity for iron and is concentrated in the earth's core.
Platinum and palladium are mined from two types of deposits:
- alluvial;
- indigenous
The difference between these varieties lies in the method of production. In bedrock deposits, the metal is contained in minerals. In such places, the extraction of the substance is carried out by processing ores - copper or nickel. Palladium is recovered as a by-product.
Placer deposits are former primary ore deposits. They have already been mined, the palladium has been released and taken the form of nuggets.
Alluvial deposits where palladium can be mined account for about 2% of global production. The largest of them are located in Russian regions - in the Urals and the Far East, as well as abroad:
- in Australia;
- Colombia;
- Canada;
- USA.
The predominant 98% of mined palladium is obtained from the depths of the earth in indigenous deposits. The main ores being mined are:
- copper-nickel;
- platinum;
- chrome.
Russia and South Africa have the status of leading countries in terms of global production of precious metal from such deposits. In honorable first place is the Norilsk Nickel enterprise - it accounts for almost half of the world's production volume. And the Bushveld complex (South Africa) owns the largest reserves of platinum group metals in the world.
Processing of ores is the main method of obtaining palladium, but not the only one. The element is recovered from recycled materials. This production method accounts for approximately 10% of total world production.
Advantages and disadvantages of metal
First of all, the demand for palladium is determined by its physical characteristics:
- Compared to platinum, it has less weight, and therefore jewelry based on it, even large ones, are not heavy at all. Moreover, its strength is much higher than that of gold. This allows it to be used as a setting for large jewelry stones. Over time, such decorations do not darken and do not lose their attractiveness.
- Another undeniable advantage of palladium is its incredible external similarity to platinum. At the same time, its cost is much lower. According to rough estimates, the cost of 1 gram of metal is 2-3 times lower than that of gold or platinum.
As part of the alloy, palladium is resistant to wear, deformation, and scratches. However, pure metal has exactly the opposite properties, and therefore is used in rare cases, for example, for making exclusive jewelry. Palladium-based wedding rings are especially in demand. It is often used instead of nickel, providing a similar effect without causing an allergic reaction.
Platinum
Platinum is one of the most expensive metals on the planet. However, its value was not immediately recognized. The metal was discovered in the 16th century in Colombia by Spanish conquistadors. The conquerors discovered unknown impurities in platinum and mistook it for low-quality silver. Then the Spanish king, fearing that the status of a “golden power” would be irretrievably lost, ordered all the platinum to be drowned. Only 50 years later, when the possibility of processing it became possible, the metal was appreciated and stood on a par with gold.
According to its properties, platinum is twice as dense as gold and has a high level of corrosion resistance, much higher. During the research, metals with similar physical and chemical properties were discovered, one of which is palladium. In addition, palladium and platinum are included in the alloy of white gold and directly determine its value.
Types of alloys and samples
Since palladium in its pure form is too soft, alloys based on it are used to make jewelry.
In Russia, two samples are legally approved - 500 and 850. The alloys used are nickel, silver and copper. 950 standard is also popular abroad. In this case, 95% palladium accounts for 5% copper or ruthenium additives. Occasionally they are replaced with nickel to provide greater strength to the alloy.
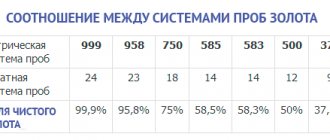
Sample correspondence table
Palladium alloys approved in Russia are prescribed in GOST. The composition and amount of ligature in each of them can be tracked using the table presented here.
| Try | Mass fraction of component, % | |||
| Palladium | Silver | Nickel | Copper | |
| 500 | 50,0 | 44,5 | 5,5 | — |
| 850 | 85,0 85,0 | 12,5 — | 2,5 — | — 15,0 |
How to spot a fake
It is almost impossible to distinguish palladium from other metals by eye.
If there is any doubt about the authenticity of the metal, it is recommended to show it to an independent jeweler appraiser. You can be sure that you are holding real jewelry in your hands if over time they have not lost their attractiveness and shine. If the jewelry begins to darken, this is definitely a fake.
Silver
The history of silver is as long as the history of gold. Since ancient times, silver has been used to mint coins. It is curious that at some point in their history, Ancient Egypt and Ancient Greece valued silver more than gold, and silver jewelry and cutlery were truly luxury items. For a long time, silver was the basis of the monetary system of Great Britain and the United States of America.
Silver is a noble, ductile and malleable metal that has a relatively low melting point (962 degrees), which makes it especially popular among jewelers. Silver, like gold, is not subject to corrosion and retains its impeccable appearance for centuries. Thanks to the skillful work of jewelers and the inclusion of precious stones, silver items not only do not decrease their value over time, but often increase it.
Tips for choosing palladium jewelry
Make sure that the jewelry is marked with an indication of the purity (500 or 850).
The silvery-white color of the metal goes better with diamonds, sapphires, amethysts, labradorite and aquamarines.
When choosing wedding rings, pay attention to the shape of the inner surface. For comfortable wearing, it should be slightly curved.
PHYSICAL PROPERTIES
| Mineral color | white |
| Stroke color | steel gray |
| Transparency | opaque |
| Shine | metal |
| Cleavage | No |
| Hardness (Mohs scale) | 4.5-5 |
| Strength | malleable |
| Kink | jagged |
| Density (measured) | 11.3 – 11.8 g/cm3 |
| Radioactivity (GRapi) | 0 |
| Magnetism | paramagnetic |
What radio components contain
In radio engineering, palladium is often found in the following parts:
- connectors;
- capacitors;
- resistors.
First of all, this concerns devices that are important in the military and space industries. In civil engineering, palladium is used only in aviation.
How to distinguish palladium from platinum in radio components
At home, distinguishing two precious metals is difficult, but possible. The easiest way is to drop a small sample into a container of nitric acid. If the metal dissolves, you have palladium.
Another method involves using a touchstone, potassium iodide and aqua regia. A metal sample is passed along the edge of the stone until a scratch is formed. Then a mixture of potassium iodide and aqua regia is poured into it. If the scratch is painted red with a brown tint, we can say that the presented sample is palladium.
Ways to isolate metal
Options:
- Electrolytic reaction. Refining involves the use of sulfuric acid, which will separate the palladium compounds, leaving the brass and copper elements intact. Aqua regia will help extract pure metal after the reaction is complete.
- Ammonia solution and hydrochloric acid also help to isolate palladium. The color of the precious metal plays an important role in the refining process. For example, brown confirms the presence of palladium in the alloy.
Also watch the video below about what else you can get palladium from:
Where does a person need
The applications of star metal in industry are varied.
- This is one of the main metals in hydrogen energy. It is used in the production of catalysts, devices for producing pure hydrogen, and sensors for determining hydrogen.
- Almost every computer, mobile phone, and high-frequency radio equipment uses alloys with platinum group metals.
- Many radio components contain precious palladium; amateurs and craftsmen extract it through refining at home.
- There are thousands of laboratories in the world, many of which require glassware made from precious metals and their alloys.
- These alloys are increasingly used in the production of medical devices and instruments.
- The use of palladium in pacemakers is welcomed by people for whom this device is vital.
- Preparations containing platinoids are increasingly used by oncologists, and palladium compounds are much more labile and less toxic than platinum compounds. The lion's share of the metal goes to dentists to “fix” our teeth.
- Palladium is hypoallergenic, which is why wedding rings or earrings made from the 950 alloy are increasingly in demand.
- Jewelers are content with 10% of mined palladium. Most often, metal is used in the form of alloys, which look great with diamonds and colorless stones.
- The main amount of mined silver metal is consumed by the automotive industry.
- Research is being conducted to extract palladium from spent fuel from nuclear power plants, and methods are being developed to obtain the metal from electrolyte solutions.
The price of palladium is steadily increasing, but the demand for the metal is still not satisfied.
Reviews
Katerina: “It just so happens that I love jewelry, especially rings. Knowing this passion of mine, my family constantly gives me something new. I also have several rings, both palladium and platinum. And I like the first ones much more. Firstly, they are lighter and do not feel much when worn. Secondly, I like their color better. Well, if you buy it yourself, palladium rings are much cheaper than platinum.”
Svetlana: “Palladium jewelry is good for everyone, except for one thing - it’s almost impossible to find them. I had to scour more than one store and online resources to find what I needed. In theory, it was possible to buy the same white gold based on palladium, but in fact very few of them are made. Basically, everything is a regular gold alloy with rhodium plating. I wish there was a better choice."
Receipt
The element is present in white gold. And an important task is to separate it from bismuth and arsenic, which, like palladium, dissolve in nitric acid.
To do this, perform the following manipulations:
- Nitrate of elements such as silver, palladium and bismuth must be evaporated to a syrupy state. In the case of palladium, this will help remove residues of various acids from them.
- Next, the mixture is diluted with purified water.
- Concentrated hydrochloric acid is added. A white sedimentary deposit, similar to cottage cheese, is formed - silver chloride. It must be separated so that the solution becomes clear.
- After this, the composition is evaporated. This removes hydrochloric acid.
- Ammonia is added to the mixture. The composition should turn blue or green. Flakes - bismuth chloride - will begin to fall out. It does not dissolve in ammonia.
- The mixture is filtered. Hydrochloric acid is added to it. As a result, palladium sulfide is formed.
- After the completion of the reaction, a yellow precipitate is formed in a transparent solution with a yellowish tint.
- Palladium sulfide must be thoroughly washed and removed from water.
- Palladium sulfide can then be reduced to its metal state. To do this, it must be melted down again.
- To give the metal a marketable appearance, palladium sulfide is best reduced to black with hydrogen sulfide. Then it needs to be fused again. After this, the palladium sulfide is granulated.